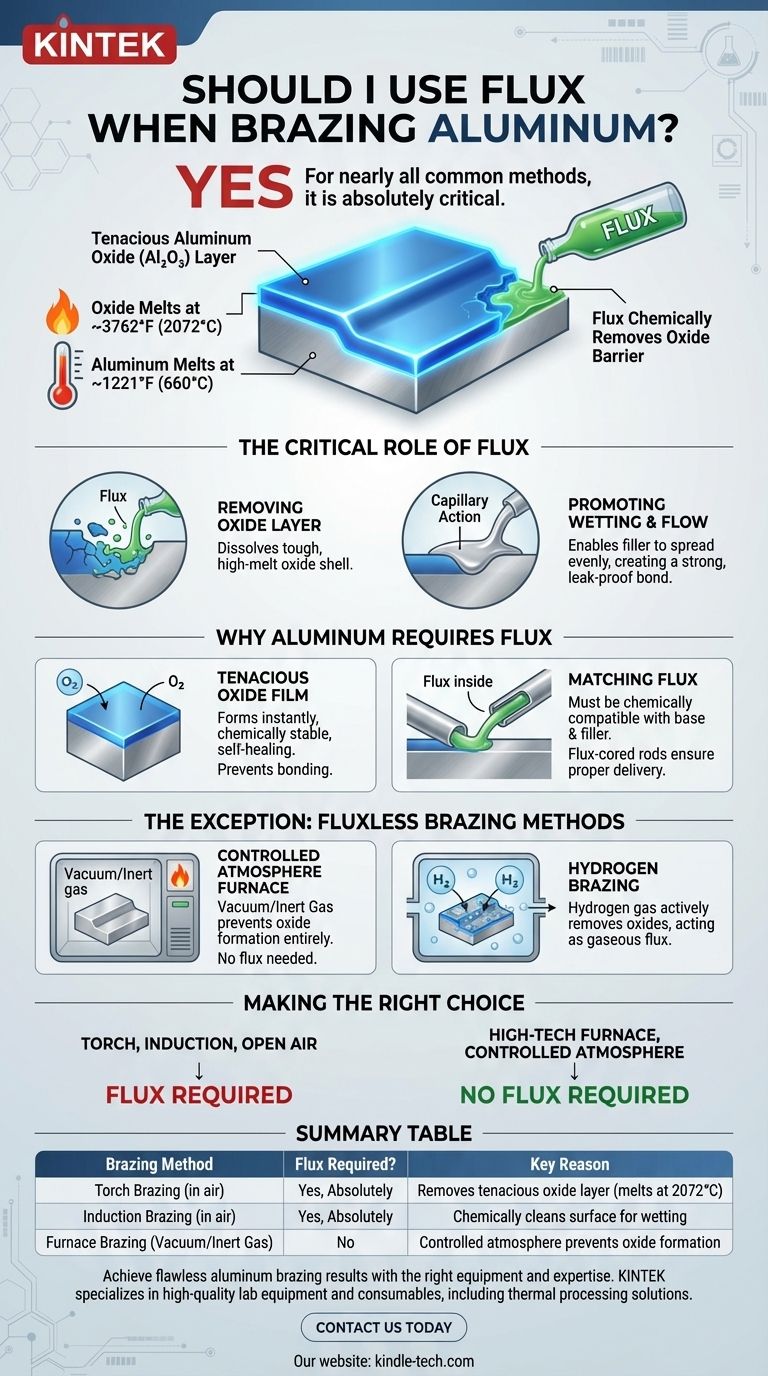
Yes, for nearly all common methods of brazing aluminum, using the correct flux is absolutely critical. Aluminum's unique chemical properties create a stubborn surface barrier that will prevent a successful braze joint from forming unless it is chemically removed by a flux. Without it, the filler metal will not bond with the base metal.
The core challenge in brazing aluminum is its tenacious, self-healing oxide layer, which has a much higher melting point than the aluminum itself. Flux is the essential chemical agent required to break down this oxide barrier, enabling the filler metal to wet the surface and create a strong, permanent bond.

The Critical Role of Flux in Brazing
Flux is not merely a cleaning agent; it is an active chemical component in the brazing process. Its primary functions are to prepare the metal surfaces and protect the joint as it forms.
Removing the Oxide Layer
All metals react with oxygen in the air to form an oxide layer. For a brazing filler metal to bond to a base metal, this layer must be completely removed at brazing temperatures.
Flux is designed to dissolve these metal oxides, exposing the pure, clean metal underneath.
Promoting Wetting and Flow
Once the oxide is gone, the flux creates a clean, protected surface. This allows the molten filler metal to "wet" the base metal, meaning it can spread evenly and thinly across the joint surfaces through capillary action.
This wetting action is what ensures a strong, continuous, and leak-proof joint.
Why Aluminum Requires a Specialized Approach
While flux is common in brazing many metals, it is non-negotiable for aluminum due to the unique nature of its oxide layer.
The Tenacious Aluminum Oxide Film
Aluminum instantly forms a layer of aluminum oxide (Al₂O₃) when exposed to air. This layer is extremely tough, chemically stable, and self-healing if scratched.
Crucially, aluminum oxide melts at about 3762°F (2072°C), while aluminum metal itself melts around 1221°F (660°C). The oxide layer will remain a solid shell, preventing the molten filler metal from ever touching the liquid aluminum beneath it.
A specialized aluminum brazing flux is required to chemically break down this high-temperature oxide shell at the much lower brazing temperature.
Matching Flux to the Material
The flux must be chemically compatible with both the aluminum base alloy and the aluminum-silicon filler metal. Using the wrong flux, or a flux intended for other metals like copper, will fail.
This is why many aluminum brazing rods are "flux-cored," ensuring the proper ratio and type of flux is delivered directly to the joint.
The Exception: Fluxless Brazing Methods
In certain industrial settings, it is possible to braze aluminum without flux, but this requires highly specialized equipment. These methods work by preventing the oxide from ever forming in the first place.
Furnace Brazing in a Controlled Atmosphere
When parts are heated in a furnace with a controlled atmosphere (such as a vacuum or an inert gas like argon), there is no oxygen available to form the oxide layer.
In this environment, the surfaces remain clean, allowing the filler metal to wet the joint without the need for a chemical flux.
Hydrogen Brazing
In some advanced applications, parts are brazed in a pure hydrogen atmosphere. At brazing temperatures, the hydrogen gas actively reacts with and removes any metal oxides on the surface.
In this case, the hydrogen atmosphere itself effectively acts as a gaseous flux.
Making the Right Choice for Your Application
Your brazing method dictates whether you need flux.
- If you are using a torch, induction, or another common heating method in open air: You absolutely must use a flux specifically designed for aluminum.
- If you are using a high-tech industrial furnace with a vacuum or controlled atmosphere: You can perform fluxless brazing, as the equipment's environment prevents oxide formation.
Ultimately, understanding the function of flux moves you from simply following steps to controlling the outcome of your work.
Summary Table:
| Brazing Method | Flux Required? | Key Reason |
|---|---|---|
| Torch Brazing (in air) | Yes, Absolutely | Removes the tenacious aluminum oxide layer (melts at 2072°C) |
| Induction Brazing (in air) | Yes, Absolutely | Chemically cleans the surface for proper filler metal wetting |
| Furnace Brazing (Vacuum/Inert Gas) | No | Controlled atmosphere prevents oxide formation entirely |
Achieve flawless aluminum brazing results with the right equipment and expertise.
Brazing aluminum presents unique challenges, but with the correct tools and knowledge, you can create strong, reliable joints every time. KINTEK specializes in providing high-quality lab equipment and consumables, including solutions for thermal processing applications.
Whether you are working in a research lab or a production environment, we can help you select the right furnace or supplies for your specific brazing needs. Let our experts guide you to success—contact us today to discuss your project!
Visual Guide
Related Products
- Conductive Carbon Fiber Brush for Static Removal and Cleaning
- Copper Foam
- Aluminum Foil Current Collector for Lithium Battery
- No Demolding Lab Infrared Press Mold for Laboratory Applications
- Warm Isostatic Press for Solid State Battery Research
People Also Ask
- Under what conditions should a carbon fiber brush be replaced? Identify Critical Failure to Ensure Performance
- What does the regular maintenance inspection of a carbon fiber brush entail? Ensure Peak Performance and Longevity
- What checks should be performed on a carbon fiber brush before use? Ensure Reliability in Your Lab Processes
- What are 3 benefits of biomass energy? Turn Waste into Renewable Power
- Is a carbon brush a good conductor of electricity? The Surprising Engineering Choice



















