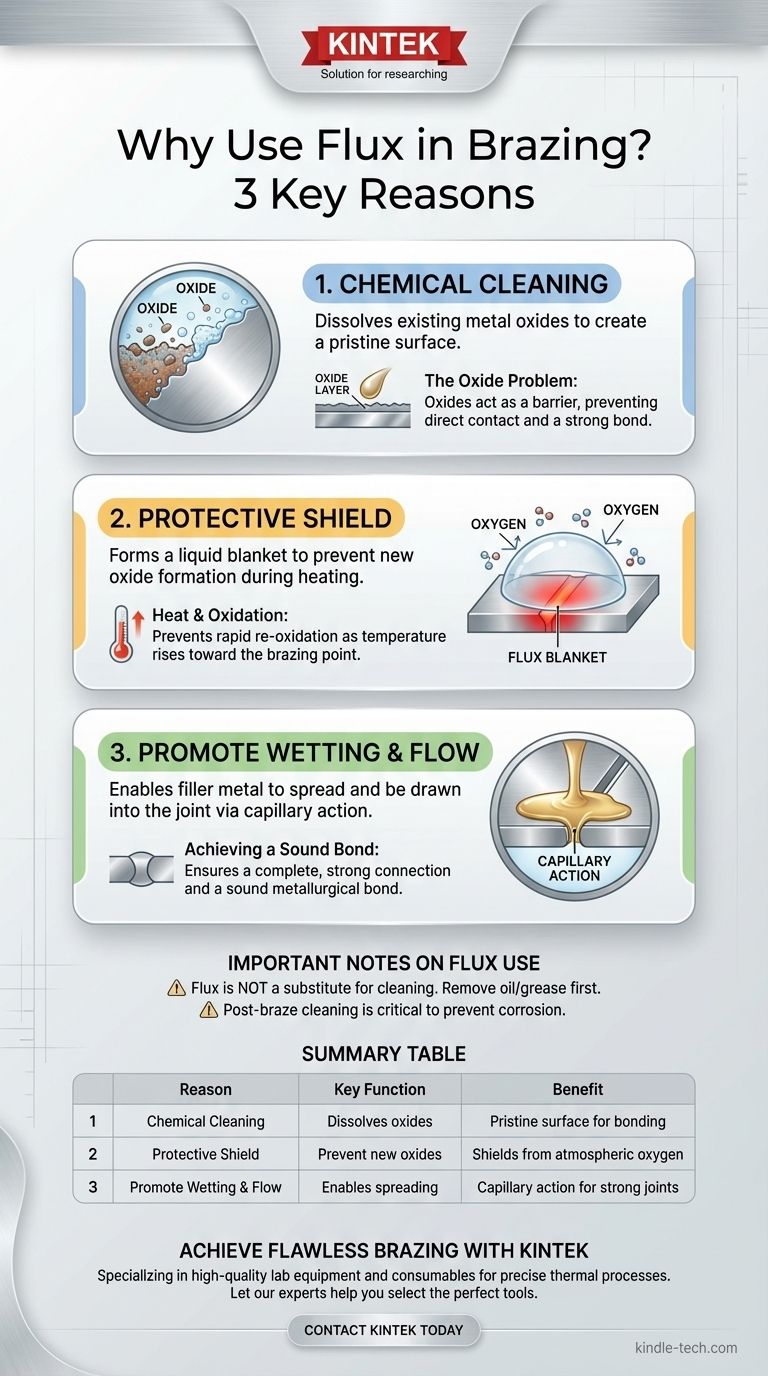
In brazing, the three primary reasons to use flux are to chemically clean the base metals, prevent the formation of new oxides during heating, and improve the wetting and flow of the filler metal. Flux is not merely a helpful additive; it is an active chemical agent that creates the necessary conditions at a microscopic level for a strong, permanent bond to form. Without it, the molten filler metal would be unable to properly adhere to the parts being joined.
Flux functions as a high-temperature chemical cleaning and shielding agent. It removes the existing metal oxides and then prevents new ones from forming, creating the pristine surface required for the filler metal to "wet" the joint and create a sound metallurgical bond.

The Core Problem: Invisible Metal Oxides
Before understanding what flux does, it's critical to understand the problem it solves. Nearly all metals react with oxygen in the air to form a thin, hard, and invisible layer of metal oxide on their surface.
What Is an Oxide Layer?
This layer is essentially a form of microscopic rust or tarnish. It forms almost instantly on a freshly cleaned metal surface, even one that appears perfectly bright and shiny to the naked eye.
Why Oxides Prevent a Good Braze
A strong brazed joint is formed when the filler metal bonds directly with the base metal. The oxide layer acts as a barrier, preventing this direct contact.
Trying to braze an oxidized surface is like trying to apply tape to a dusty wall—it will stick to the dust, not the wall itself, resulting in a weak and unreliable bond.
How Flux Solves the Oxide Problem
Flux is a chemical compound designed to be active at brazing temperatures. Its role is multifaceted, addressing the oxide problem at every stage of the heating process.
The Chemical Cleaning Action
As the flux is heated, it melts and becomes chemically active. Its primary function is to dissolve the existing metal oxides on the surface of the base metals, clearing the way for the filler metal.
This is a true chemical cleaning process that prepares the surface on a level that mechanical cleaning (like sanding or grinding) cannot achieve alone.
The Protective Shield
Once the flux has cleaned the surface, it forms a liquid blanket over the entire joint area. This blanket shields the hot, clean base metal from oxygen in the surrounding atmosphere.
This prevents the rapid re-oxidation that would otherwise occur as the metal's temperature rises toward the brazing point.
Promoting Wetting and Flow
With a perfectly clean and protected surface, the molten filler metal can "wet" the base metal. Wetting is the ability of a liquid to spread out over a solid surface.
Good wetting allows the filler metal to be drawn evenly into the tight gap of the joint through a process called capillary action, ensuring a complete, void-free, and strong connection.
Understanding the Trade-offs and Limitations
While essential, flux is a powerful chemical that must be used correctly. Understanding its limitations is key to achieving consistent, high-quality results.
Flux Is Not a Substitute for Cleaning
Flux is designed to remove microscopic oxides, not heavy contaminants. It will not remove oil, grease, or excessive dirt. Proper mechanical and chemical pre-cleaning is still a mandatory first step.
The Risk of Post-Braze Corrosion
Most fluxes are corrosive by nature, which is what makes them effective. If excess flux is not thoroughly removed after brazing, it can absorb moisture from the air and cause severe corrosion, weakening the joint and the base metal over time.
The Exception: Fluxless Brazing
In some industrial processes, like furnace brazing, a flux is not used. Instead, the parts are heated inside a sealed furnace with a controlled atmosphere of inert or reducing gases.
This special atmosphere prevents oxygen from ever contacting the parts, thus eliminating the need for a chemical flux to remove or prevent oxides. This highlights that the core goal is always oxide control, whether by chemical or atmospheric means.
Making the Right Choice for Your Goal
The type of flux must be chemically compatible with both the base metal and the filler metal you are using. Always match these components for a successful outcome.
- If your primary focus is general-purpose fabrication or repair: Select a standard, general-purpose flux that is specifically rated for your base metal (e.g., copper, steel, brass) and the temperature range of your filler alloy.
- If your primary focus is joining corrosion-sensitive materials: You must use a flux formulated for that specific material (like stainless steel) and implement a rigorous post-braze cleaning procedure.
- If your primary focus is high-volume, clean production: Investigating controlled atmosphere furnace brazing may be a viable fluxless alternative that improves consistency and eliminates the cleaning step.
Ultimately, using the right flux correctly transforms brazing from a simple act of melting metal into a controlled chemical process that ensures a reliable bond.
Summary Table:
| Reason to Use Flux | Key Function |
|---|---|
| Chemical Cleaning | Dissolves existing metal oxides to create a pristine surface. |
| Protective Shield | Forms a liquid blanket to prevent new oxide formation during heating. |
| Promotes Wetting & Flow | Enables filler metal to spread and be drawn into the joint via capillary action. |
Achieve flawless brazing results with the right equipment and consumables. KINTEK specializes in high-quality lab equipment and consumables, serving all your laboratory needs. Whether you are involved in R&D, quality control, or specialized fabrication, our products support precise thermal processes. Let our experts help you select the perfect tools for your application. Contact KINTEK today to discuss your specific requirements and enhance your brazing process.
Visual Guide
Related Products
- Special Heat Press Mold for Lab Use
- Assemble Square Lab Press Mold for Laboratory Applications
- Cylindrical Press Mold for Lab Applications
- High Purity Zinc Foil for Battery Lab Applications
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
People Also Ask
- What is the standard temperature for heat press? Master the Perfect Settings for Durable Transfers
- Is it fitting the mould or mold? A Guide to Correct Spelling by Region
- What is a hot hydraulic press? Harness Heat and Pressure for Advanced Manufacturing
- What are the parts of a press mold? A Guide to Punch, Die, and Key Components
- How to use a press mold? Master the Art of Creating Consistent Ceramic Forms



















