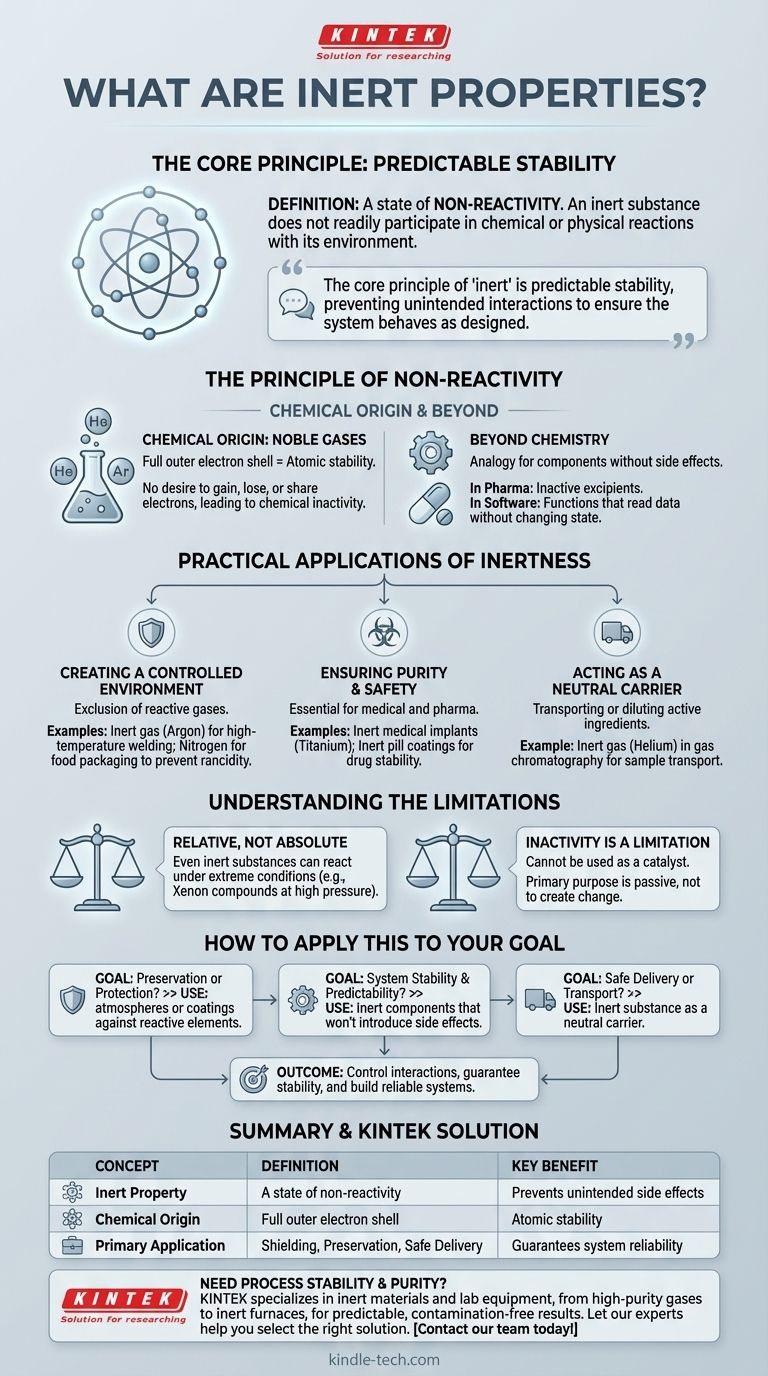
In simple terms, an inert property describes a state of non-reactivity. A substance or component that is inert does not readily participate in chemical or physical reactions when it comes into contact with other elements in its environment. The classic example comes from chemistry, where inert gases like helium and argon are famously stable because their atomic structure discourages the formation of chemical bonds.
The core principle of "inert" is predictable stability. An inert element is intentionally included in a system to prevent unintended interactions, ensuring the system behaves exactly as designed without interference.

The Principle of Non-Reactivity
The concept of being inert is fundamental across many scientific and engineering disciplines. It originates from chemistry but is applied as a powerful analogy in fields ranging from materials science to software engineering.
The Chemical Origin: A Stable Foundation
The textbook definition of inertness comes from the behavior of noble gases. These elements have a full outer shell of electrons, which is the most stable configuration for an atom.
Because they are not seeking to gain, lose, or share electrons, they do not easily form chemical bonds with other atoms. This electronic stability is the source of their chemical inactivity.
Why This Stability Matters
This lack of reactivity is not a flaw but a crucial and desirable feature. When you introduce an inert substance into a process, you can be confident that it will not cause unexpected side effects.
It serves as a neutral, predictable background element, allowing other, more reactive components to interact in a controlled manner.
"Inert" Beyond Chemistry
The term has been adopted to describe any component that does not produce a side effect. In pharmacology, the inactive ingredients in a pill are called excipients and are chosen for their inert properties.
In software, an "inert" function might be one that reads data but doesn't change the state of the system, guaranteeing it won't introduce bugs elsewhere.
Practical Applications of Inertness
Harnessing the property of inertness is critical for safety, preservation, and purity in countless industrial and scientific processes.
Creating a Controlled Environment
Many processes require the exclusion of reactive gases like oxygen. In high-temperature welding, an inert gas like argon is used to shield the molten metal, preventing oxidation that would weaken the weld.
Similarly, nitrogen is often used to package foods like potato chips. It displaces oxygen, preventing the fats from going rancid and keeping the product fresh.
Ensuring Purity and Safety
In pharmaceutical manufacturing, inert materials are essential. Medical implants are made from inert materials like titanium or certain polymers to ensure they do not react with the tissues of the human body.
The coatings on pills must be inert to prevent them from reacting with the active drug, ensuring the medication's stability and correct dosage delivery.
Acting as a Neutral Carrier
Inert substances are often used as a vehicle to transport or dilute a more volatile or concentrated active ingredient.
In analytical chemistry, an inert gas like helium is used in a gas chromatograph to carry the sample through the machine without interfering with the measurement.
Understanding the Limitations
While incredibly useful, the concept of inertness is not absolute and comes with its own considerations.
Inertness is Relative, Not Absolute
Even the most inert substances can be forced to react under extreme conditions. For example, while once considered completely inert, chemists have successfully created compounds using noble gases like xenon under very high pressure and temperature.
In practical terms, a material considered inert for one application may be reactive in another, more demanding environment.
The Inactivity Itself is a Limitation
The primary strength of an inert substance—its inactivity—is also its main limitation. You cannot use an inert material as a catalyst, because a catalyst must participate in a reaction.
Its purpose is to be passive. If your goal is to create a chemical change, an inert component is, by definition, the wrong tool for the job.
How to Apply This to Your Goal
Your need for an inert material depends entirely on what you are trying to achieve within your system.
- If your primary focus is preservation or protection: Use inert atmospheres or coatings to build a shield against reactive elements like oxygen, thereby preventing corrosion, spoilage, or degradation.
- If your primary focus is system stability and predictability: Choose inert components or ingredients that will not introduce side effects or interfere with the primary function of your system.
- If your primary focus is safe delivery or transport: Utilize an inert substance as a neutral carrier for a sensitive, concentrated, or reactive active ingredient.
Ultimately, understanding inertness empowers you to control interactions, guarantee stability, and build more reliable and predictable systems.
Summary Table:
| Concept | Definition | Key Benefit |
|---|---|---|
| Inert Property | A state of non-reactivity | Prevents unintended side effects |
| Chemical Origin | Full outer electron shell (e.g., Noble Gases) | Atomic stability |
| Primary Application | Shielding, Preservation, Safe Delivery | Guarantees system reliability |
Need to ensure process stability and purity? KINTEK specializes in providing the inert materials and lab equipment—from high-purity gases to inert atmosphere furnaces—that your laboratory relies on for predictable, contamination-free results. Let our experts help you select the right solution for your application. Contact our team today to discuss your needs!
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- High-Purity Titanium Foil and Sheet for Industrial Applications
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- High Purity Zinc Foil for Battery Lab Applications
- Vacuum Dental Porcelain Sintering Furnace
People Also Ask
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- What is the role of an atmosphere-controlled tube furnace in Cu-Mo sintering? Achieve High-Purity Densification
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety



















