In short, a super-sealed electrolytic cell is primarily used for sensitive electrochemical experiments where environmental control is paramount. Its common applications include electrochemical synthesis, battery testing, corrosion research, environmental monitoring, and various energy-related studies.
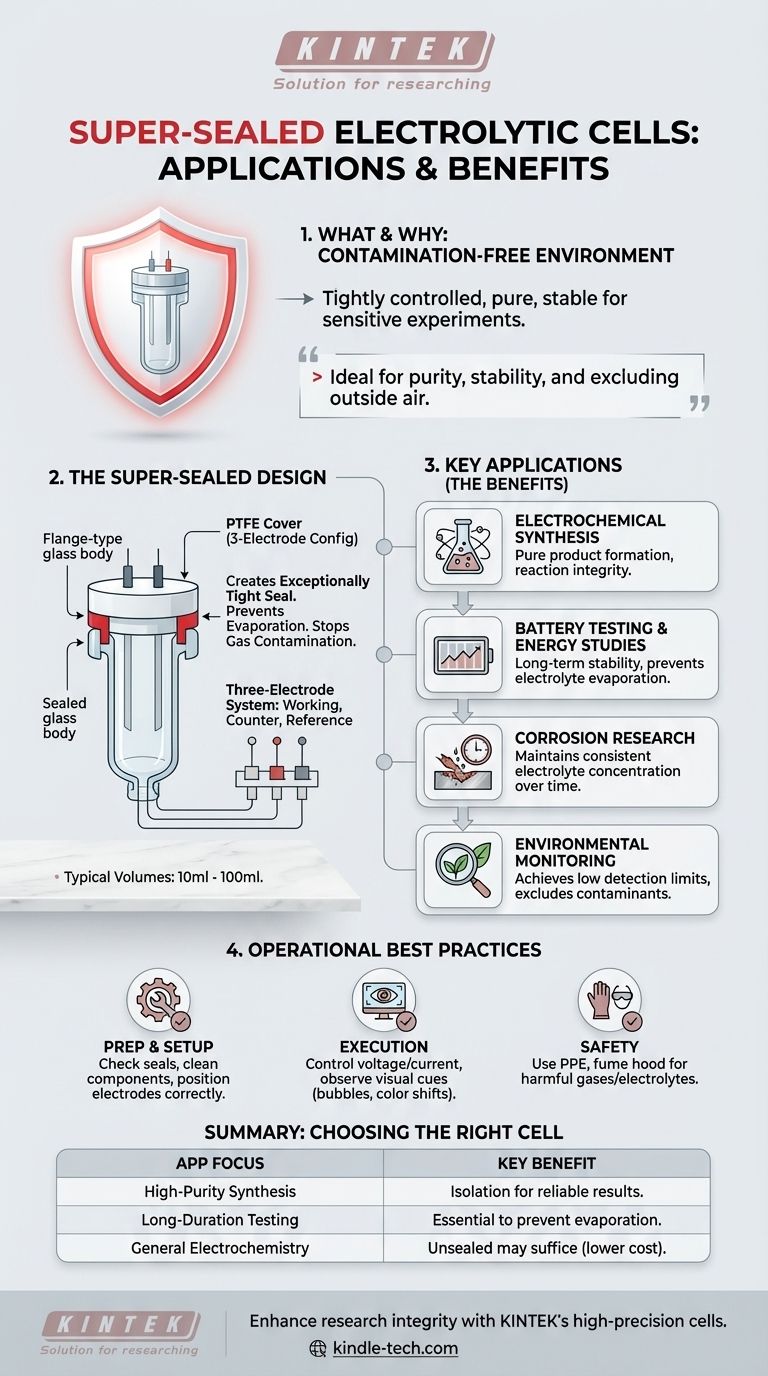
The defining feature of a super-sealed electrolytic cell is its ability to create a tightly controlled, contamination-free environment. This makes it the ideal choice for experiments where purity, stability, and the exclusion of outside air are critical for obtaining accurate and reproducible results.

Understanding the Core Design and Function
A super-sealed cell is not just a container; it is a precision instrument designed for exacting electrochemical work. Its value lies in its construction, which directly enables its specialized applications.
The Three-Electrode System
These cells are typically configured for a three-electrode system. This setup includes a working electrode (where the reaction of interest occurs), a counter electrode (to complete the electrical circuit), and a reference electrode (which provides a stable potential to measure against).
The "Super-Sealed" Advantage
The cell's name comes from its construction. It uses a flange-type glass body and a Polytetrafluoroethylene (PTFE) cover. These two components are pressed together by a flange, creating an exceptionally tight seal.
This design prevents the electrolyte from evaporating, stops atmospheric gases like oxygen from contaminating the solution, and ensures that any gases produced during the experiment can be contained or managed.
Key Physical Specifications
These cells are designed for small-scale, precise lab work, with typical volumes ranging from 10ml to 100ml.
The PTFE cover usually has a standard configuration of three larger holes (around 6.2mm) for the electrodes and two smaller holes (around 3.2mm) for tasks like purging the solution with an inert gas.
A Closer Look at Key Applications
The sealed design is what makes this cell indispensable for specific fields of research. The need for environmental isolation is the common thread.
Electrochemical Synthesis & Energy Studies
When synthesizing a new compound or studying charge/discharge cycles, any impurity can alter the reaction pathway or final product. The sealed environment ensures the reaction proceeds exactly as intended.
Battery Testing and Corrosion Research
These experiments often run for hours or even days. An unsealed cell would suffer from electrolyte evaporation, changing its concentration and skewing the results. The super-sealed design guarantees long-term stability for reliable data.
Environmental Monitoring
When measuring trace amounts of pollutants, accuracy is everything. A sealed cell prevents contaminants from the lab air from entering the sample, which is critical for achieving low detection limits and trustworthy measurements.
Operational Best Practices and Pitfalls
Proper handling is crucial to leveraging the benefits of a super-sealed cell and ensuring both safety and data integrity. Mistakes in setup can negate the advantages of the sealed design.
Pre-Experiment Preparation
Before starting, always check that all components are present and the seals are undamaged. Clean the glass body and electrodes thoroughly with appropriate solvents and rinse with distilled water.
When installing the electrodes, ensure they are positioned correctly and that all wiring is secure. Finally, carefully pour in the filtered electrolyte to avoid contamination.
During the Experiment
Correctly connect the cell to your potentiostat or power supply. Carefully control the experimental parameters like voltage and current, and monitor the process for any abnormalities.
Observe the experiment for visual cues, such as changes on the electrode surface, gas bubbles, or shifts in solution color. These can provide valuable insights into the reaction.
Critical Safety Considerations
Always familiarize yourself with the equipment's operation manual. When working with corrosive electrolytes or reactions that produce harmful gases, use appropriate personal protective equipment (PPE) and operate within a well-ventilated fume hood.
Making the Right Choice for Your Research
Choosing the correct cell depends entirely on the requirements of your experiment.
- If your primary focus is high-purity synthesis or trace analysis: The isolation provided by a super-sealed cell is non-negotiable for reliable results.
- If your primary focus is long-duration testing (corrosion or battery cycling): A super-sealed cell is essential to prevent electrolyte evaporation and maintain stable conditions.
- If your primary focus is general electrochemistry or educational demonstration: A simpler, unsealed glass cell may be sufficient and more cost-effective if absolute environmental control is not required.
Ultimately, this cell is a specialized tool chosen when the integrity of your electrochemical system cannot be compromised.
Summary Table:
| Application Area | Key Benefit of Super-Sealed Design |
|---|---|
| Electrochemical Synthesis | Prevents atmospheric contamination for pure product formation. |
| Battery & Cell Testing | Ensures long-term stability by preventing electrolyte evaporation. |
| Corrosion Research | Maintains consistent electrolyte concentration over extended periods. |
| Environmental Monitoring | Achieves low detection limits by excluding airborne contaminants. |
| Energy Studies | Provides a controlled environment for accurate reaction pathway analysis. |
Need a contamination-free environment for your electrochemical research?
KINTEK specializes in high-precision lab equipment, including super-sealed electrolytic cells designed for sensitive applications like battery testing, corrosion studies, and environmental analysis. Our cells ensure the purity and stability you need for accurate, reproducible results.
Contact our experts today to find the perfect electrolytic cell for your laboratory's specific requirements and enhance the integrity of your research.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Flat Corrosion Electrolytic Electrochemical Cell
People Also Ask
- What role does a three-electrode electrolytic cell system play in testing perovskite oxides? Maximize Catalytic Data.
- What advantages do split cells and ion-exchange membranes offer in gold electrowinning? Boost Efficiency & Purity
- What role does a partitioned Electrolytic Cell play in the recovery of nickel-based superalloys? Expert Insights
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What are the complete post-experiment procedures for a flat plate corrosion electrolytic cell? A Step-by-Step Guide to Reliable Results
- What are the construction materials and design features of the thin-layer spectroelectrochemical cell body? Explored
- How does a two-electrode DC system influence coating quality? Achieve Dense Trivalent Chromium on 304L Stainless Steel
- Why is a platinum (Pt) cylindrical mesh selected as the anode in Zn-Ni alloy electrolytic cells? Explained



















