When selecting a surface hardening method, nitriding stands out for its ability to enhance wear resistance and fatigue life without requiring high temperatures that cause distortion. The primary types of nitriding are gas, salt bath (liquid), and plasma (ion) nitriding. While all three processes achieve surface hardening by diffusing nitrogen into a steel or alloy part, they use fundamentally different media and offer distinct advantages in control, cost, and application.
The choice between gas, salt bath, and plasma nitriding is not a matter of which is "best," but which process provides the optimal balance of metallurgical control, production volume, and cost for your specific engineering requirement.

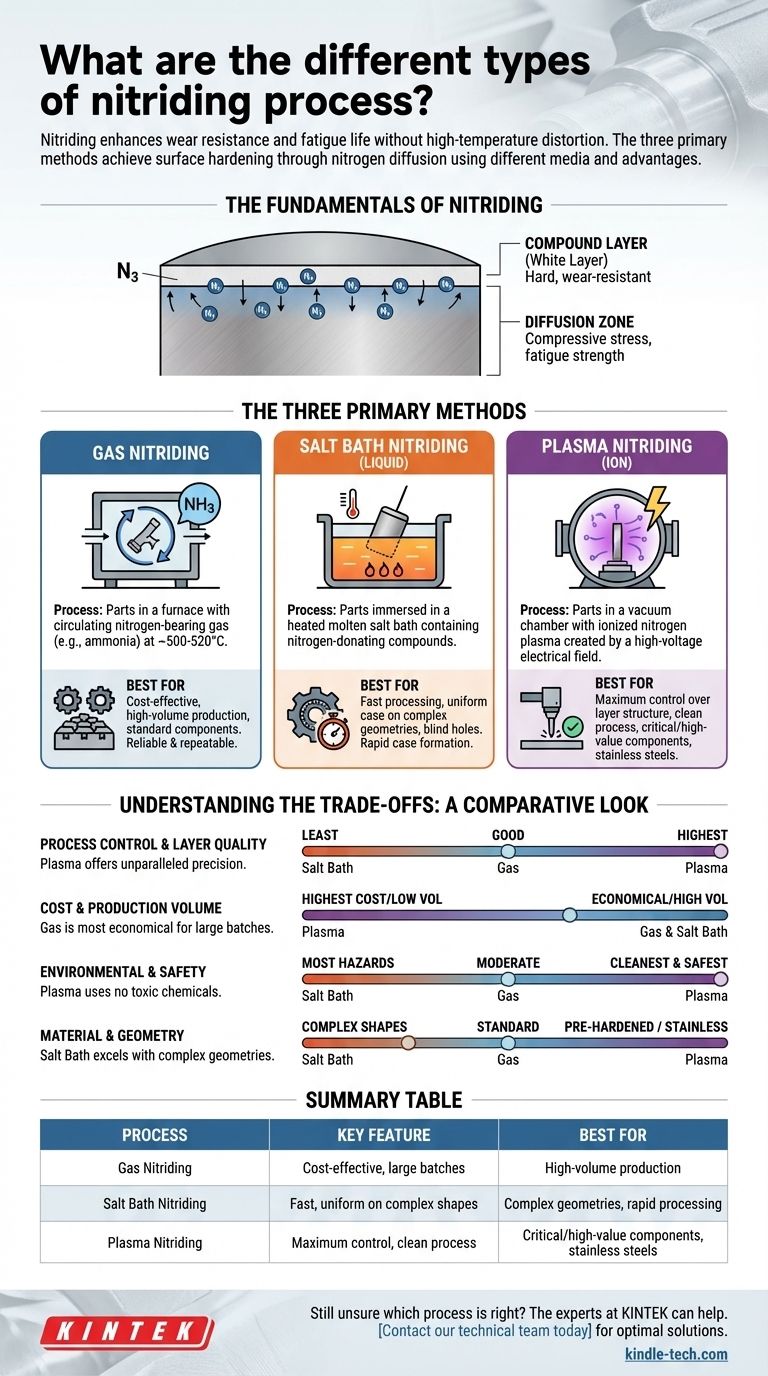
The Fundamentals of Nitriding
To choose a process, you must first understand the goal. Nitriding isn't just one thing; it creates a structured surface with distinct zones.
What is Nitriding?
Nitriding is a thermochemical case-hardening process that diffuses nitrogen atoms into the surface of a metal. This creates hard nitride compounds with the base metal and its alloying elements, dramatically increasing surface hardness.
The Goal: Compound Layer and Diffusion Zone
The process forms two primary layers. The outermost compound layer (or "white layer") is extremely hard and provides exceptional wear and corrosion resistance.
Beneath that, the diffusion zone is where nitrogen atoms have penetrated the material's crystal lattice, creating compressive stresses. This zone is responsible for the significant increase in fatigue strength.
The Three Primary Nitriding Methods
Each method uses a different medium to deliver nitrogen to the component's surface, which directly impacts the process characteristics.
Gas Nitriding
This is the most traditional and widely used method. Parts are placed in a furnace with a circulating atmosphere of nitrogen-bearing gas, most commonly ammonia (NH3).
At a typical temperature of 500-520°C, the ammonia dissociates on the steel's surface, releasing active nitrogen atoms that diffuse into the part.
The primary benefit of gas nitriding is its cost-effectiveness for treating large batches of components. It is a well-understood and highly repeatable process.
Salt Bath Nitriding (Liquid Nitriding)
This method involves immersing parts in a heated, molten salt bath containing nitrogen-donating compounds. These are typically cyanide-cyanate based salts.
The process is often referred to by trade names like Tenifer or Melonite. It is faster than gas nitriding and excellent for producing a uniform case on complex shapes.
Many salt bath processes are actually nitrocarburizing, as they introduce both nitrogen and a small amount of carbon into the surface for enhanced properties.
Plasma Nitriding (Ion Nitriding)
Plasma nitriding is the most technologically advanced method. Parts are placed in a vacuum chamber, which is then backfilled with a precise mixture of gases, primarily nitrogen.
A high-voltage electrical field is applied, creating an ionized gas or plasma around the component. These nitrogen ions are accelerated and bombard the part's surface, heating it and providing the active nitrogen for diffusion.
This method offers unparalleled control over the structure and composition of the nitrided layers.
Understanding the Trade-offs: A Comparative Look
No single process is superior in all situations. The right choice depends on balancing performance needs with practical constraints.
Process Control and Layer Quality
Plasma nitriding offers the highest degree of control. By precisely managing the gas mixture, pressure, and electrical parameters, you can selectively control the growth of the compound layer. This is critical for applications where a brittle white layer is undesirable.
Gas nitriding offers good control over case depth but less control over the compound layer's phase composition compared to plasma. Salt bath nitriding offers the least process control.
Cost and Production Volume
Gas nitriding is the most economical for high-volume production. The equipment and consumables are relatively inexpensive, and large furnaces can process many parts at once.
Salt bath nitriding is also well-suited for high-volume work. Plasma nitriding has the highest initial equipment cost and is typically better for smaller batches or individual high-value components.
Environmental and Safety Impact
Plasma nitriding is the cleanest and safest process. It uses no toxic chemicals and produces no hazardous byproducts.
Gas nitriding uses large quantities of ammonia, which is a toxic and flammable gas requiring careful handling. Salt bath nitriding presents the most significant hazards due to the use of high-temperature, toxic cyanide salts and the resulting disposal challenges.
Material and Geometry Considerations
Plasma's lower processing temperatures make it ideal for pre-hardened steels, as it can nitride below the material's tempering temperature, preserving core strength. It's also uniquely effective for stainless steels.
Salt bath nitriding excels at treating parts with very complex geometries, blind holes, and small orifices, as the liquid ensures complete and uniform surface contact.
Selecting the Right Process for Your Application
Your final decision should be guided by your project's most critical factor.
- If your primary focus is cost-effective treatment of large batches: Gas nitriding is the industry standard and offers a reliable balance of properties.
- If your primary focus is maximum control and performance for critical components: Plasma nitriding offers unparalleled precision over the case structure, making it ideal for high-value applications.
- If your primary focus is speed and treating parts with complex geometries: Salt bath nitriding provides rapid case formation but requires careful management of hazardous materials.
By understanding these core differences, you can select the nitriding process that aligns perfectly with your engineering goals and operational capabilities.
Summary Table:
| Process | Key Feature | Best For |
|---|---|---|
| Gas Nitriding | Cost-effective, large batches | High-volume production, standard components |
| Salt Bath Nitriding | Fast, uniform on complex shapes | Complex geometries, rapid processing |
| Plasma Nitriding | Maximum control, clean process | Critical/high-value components, stainless steels |
Still unsure which nitriding process is right for your components? The experts at KINTEK are here to help. We specialize in providing lab equipment and consumables for material testing and analysis, including surface hardening processes. We can help you analyze your specific requirements for material, geometry, and performance to recommend the optimal solution. Contact our technical team today to discuss your project and ensure you achieve the perfect surface properties for your application.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vertical Laboratory Tube Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What materials are used in a vacuum furnace? Selecting the Right Hot Zone for Your Process
- What are vacuum furnaces used for? Unlock Ultimate Material Purity and Performance
- How to vacuum out a furnace? A Step-by-Step Guide to Safe DIY Maintenance
- Why do you vacuum for heat treatment? Achieve Flawless, High-Performance Metal Components



















