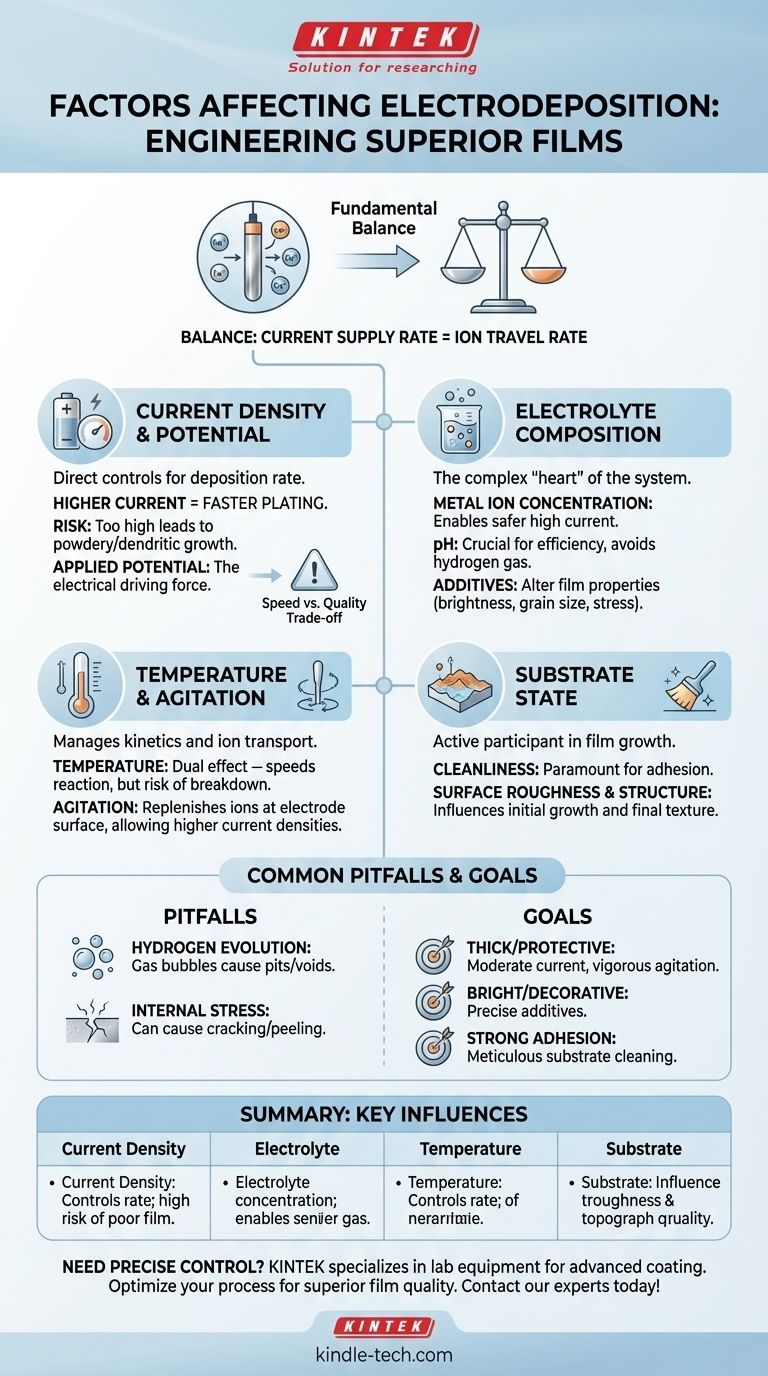
At its core, the quality and characteristics of a film created by electrodeposition are controlled by four primary factors: current density, electrolyte composition, temperature, and the physical state of the substrate. These variables work in concert to dictate the rate of deposition, the structure of the final coating, and its adhesion to the surface.
The success of any electrodeposition process hinges on a fundamental balance: the rate at which you supply electrical current must be matched by the rate at which metal ions can travel through the electrolyte to the electrode surface. When this balance is disrupted, coating quality deteriorates rapidly.

The Role of Current and Potential
The electrical parameters you apply are the most direct controls you have over the deposition rate. They act as the engine driving the entire process.
Current Density: The Pace of Deposition
Current density, measured in amperes per square centimeter (A/cm²), directly governs how quickly the metal film builds up. A higher current density forces more metal ions to deposit per unit of time, increasing the plating speed.
The Risk of Exceeding the Limit
However, there is a critical limit. If the current density is too high, ions are consumed at the electrode faster than they can be replenished from the electrolyte. This leads to poor-quality, powdery, or dendritic (tree-like) growths instead of a smooth, dense film.
Applied Potential as the Driving Force
The applied potential (voltage) is the electrical force that drives the current. In many systems, you control the potential and measure the resulting current. The required potential is influenced by the entire system, including the electrolyte's resistance and the specific reactions occurring at both electrodes.
The Electrolyte: The Heart of the System
The electrolyte bath is far more than just a source of metal. Its specific chemistry is arguably the most complex and influential aspect of the process.
Metal Ion Concentration
A higher concentration of the desired metal ions in the solution allows for higher current densities to be used safely. It ensures a ready supply of ions is available near the electrode surface, preventing depletion and maintaining coating quality at faster deposition rates.
The Critical Impact of pH
The pH of the electrolyte bath is crucial. It can affect the chemical form of the metal ions and influence the efficiency of the deposition. An incorrect pH can promote unwanted side reactions, most notably the evolution of hydrogen gas.
Additives and Brighteners
Small quantities of specific organic or inorganic compounds are often added to the electrolyte. These agents can dramatically alter the film's properties, controlling grain size, increasing brightness, improving leveling, and relieving internal stress in the deposit.
Environmental and Physical Factors
The physical conditions of the deposition environment play a significant role in managing the transport of ions and the overall reaction kinetics.
Temperature's Dual Effect
Increasing the temperature generally increases the conductivity of the electrolyte and speeds up reaction rates, which can be beneficial. However, excessively high temperatures can also accelerate the breakdown of additives or increase the rate of unwanted side reactions.
Agitation and Mass Transport
Stirring the electrolyte, either mechanically or through solution flow, is critical for achieving uniform coatings. Agitation ensures that the layer of solution next to the electrode is constantly replenished with metal ions, enabling the use of higher current densities without sacrificing quality.
The Substrate's Influence
The surface being coated, or the substrate, is not a passive participant. Its cleanliness is paramount for good adhesion. Furthermore, the material's surface roughness and crystal structure can influence the initial stages of film growth and the final texture of the coating.
Common Pitfalls to Avoid
Achieving a perfect coating requires navigating a series of common trade-offs and potential failure modes.
Speed vs. Quality
The most fundamental trade-off is between the speed of deposition and the quality of the resulting film. Pushing for faster plating by increasing current density almost always comes at the cost of smoothness, density, and adhesion if other factors are not adjusted to compensate.
Hydrogen Evolution
A common and highly disruptive side reaction is the reduction of water or H+ ions to form hydrogen gas. This process consumes current that would otherwise go to metal deposition, lowering efficiency. Worse, the gas bubbles that form on the surface create pits and voids, severely compromising the integrity of the coating.
Internal Stress
As a film is deposited, it can develop internal stresses, either compressive or tensile. High levels of stress can cause the coating to crack, peel away from the substrate, or even deform the substrate itself. This is often managed through careful selection of additives and operating conditions.
Making the Right Choice for Your Goal
By deliberately controlling these factors, you can engineer a film with specific properties.
- If your primary focus is a thick, protective coating: Prioritize moderate current density with vigorous agitation to ensure a steady, uninterrupted supply of metal ions.
- If your primary focus is a bright, decorative finish: Your control over the electrolyte, particularly the precise blend of additives and brighteners, will be the most critical factor.
- If your primary focus is strong adhesion for performance applications: Meticulous substrate cleaning and surface preparation are non-negotiable prerequisites for success.
By systematically controlling these interconnected variables, you can move from simply coating a surface to truly engineering a material with precisely the properties your application demands.
Summary Table:
| Factor | Key Influence on Electrodeposition |
|---|---|
| Current Density | Controls deposition rate; too high causes poor, powdery films. |
| Electrolyte Composition | Determines metal ion supply, pH balance, and additive effects. |
| Temperature | Affects reaction kinetics and electrolyte conductivity. |
| Substrate State | Influences initial film growth, adhesion, and final texture. |
Need precise control over your electrodeposition process? KINTEK specializes in lab equipment and consumables for advanced material coating applications. Our solutions help you optimize current density, electrolyte composition, and temperature for superior film quality, adhesion, and performance. Contact our experts today to discuss how we can support your laboratory's specific coating challenges!
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What are the advantages of using HFCVD for BDD electrodes? Scaling Industrial Diamond Production Efficiently
- How are reactants introduced into the reaction chamber during a CVD process? Mastering Precursor Delivery Systems
- How does PACVD equipment improve DLC coatings? Unlock Low Friction and High Heat Resistance
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating
- How is something diamond coated? A Guide to CVD Growth vs. Plating Methods



















