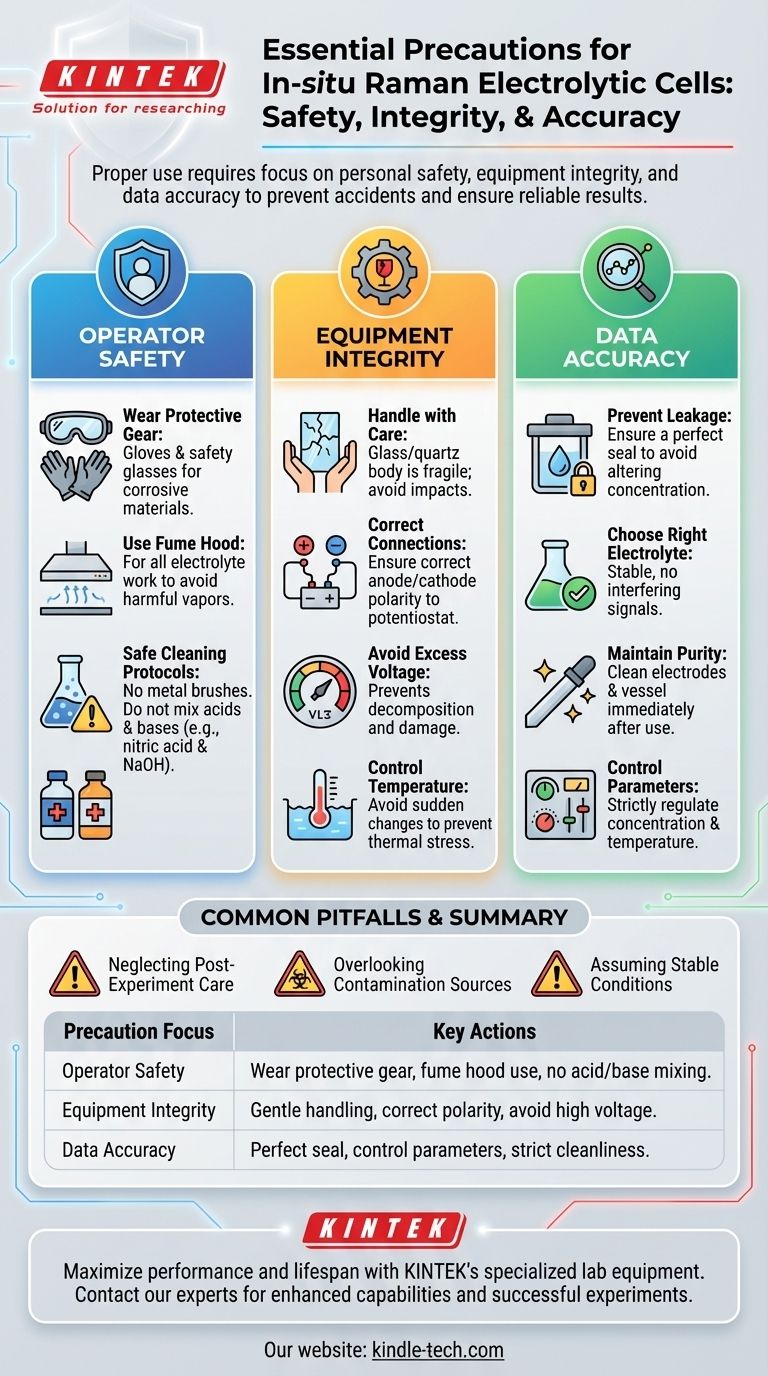
Properly using an in-situ Raman electrolytic cell requires a strict focus on three key areas: personal safety, equipment integrity, and data accuracy. The most critical precautions include wearing protective gear, handling the fragile cell body with care, ensuring correct electrical polarity, and preventing any electrolyte leakage which could compromise both safety and experimental results.
An in-situ Raman cell is a powerful tool for observing electrochemical processes in real-time. However, its effectiveness hinges on meticulous preparation and handling. The primary goal of these precautions is not just to prevent accidents, but to eliminate variables that could invalidate your scientific data.

Prioritizing Operator Safety
Your first responsibility is to ensure a safe working environment for yourself and others in the lab. This involves careful handling of both chemical and physical hazards.
Handling Corrosive Materials
Always wear appropriate protective gloves and safety glasses when working with electrolytes, many of which can be corrosive or toxic.
Perform all work involving electrolytes inside a fume hood to prevent the inhalation of potentially harmful gases or vapors generated during the experiment.
Safe Cleaning Protocols
Never use metal brushes or other abrasive tools to clean the cell. These can easily scratch the glass or quartz surfaces, which can interfere with optical measurements and weaken the cell's structure.
Crucially, do not mix acids and bases (e.g., nitric acid and sodium hydroxide) during cleaning. This combination can produce a dangerous exothermic reaction, posing a significant safety risk.
Ensuring Equipment Integrity
The electrolytic cell is a sensitive and often expensive piece of equipment. Protecting it from damage is essential for long-term use and reliable performance.
Handle with Care
The cell body is typically made of glass or quartz, making it fragile. Always handle it gently to avoid impacts with hard objects, which could cause it to crack or break.
Correct Electrical Connection
Ensure the anode and cathode are connected correctly to the potentiostat. A reverse connection can lead to unintended reactions and potential damage to your electrodes.
Avoid applying excessively high voltage. This can cause the electrolyte to decompose, damage the electrode surfaces, and generate unwanted side products.
Control the Temperature
While materials like quartz are heat-resistant, avoid sudden or extreme temperature changes. This can create thermal stress and lead to cracking.
If using a constant temperature water bath, ensure its controller is functioning correctly to maintain a stable and precise temperature.
Guaranteeing Data Accuracy
The ultimate goal is to collect high-quality, reproducible data. Several operational precautions are directly linked to the integrity of your results.
Prevent All Electrolyte Leakage
The cell must be perfectly sealed. Even a minor leak can alter the electrolyte concentration, affect the reaction, and compromise your results, in addition to being a safety hazard.
Choose the Right Electrolyte
Select an electrolyte that is stable within your experimental window and does not produce interfering Raman signals or unwanted side reactions with your electrodes.
Maintain Electrode Purity
Clean the electrodes and reaction vessel immediately after each experiment. This prevents residue buildup that could contaminate subsequent experiments and alter surface chemistry.
Control Experimental Parameters
Strictly control variables like electrolyte concentration and temperature. Fluctuations in these parameters can directly influence reaction kinetics and the resulting Raman spectra, making your data difficult to interpret or reproduce.
Common Pitfalls That Compromise Results
Beyond basic safety and handling, certain oversights are common sources of experimental failure. Being aware of them is critical for obtaining reliable data.
Neglecting Post-Experiment Care
Failing to clean and properly dry the cell after use is a primary source of contamination. For long-term storage, disassemble the cell and store its components in a dry, protected environment.
Overlooking Contamination Sources
Impurities can enter the cell from the electrolyte, the atmosphere, or from contaminated handling tools. Always maintain a high standard of cleanliness for all components.
Assuming Stable Conditions
Do not assume parameters like temperature will remain constant without active control. Regularly check your equipment to ensure stability throughout the experiment's duration.
Making the Right Choice for Your Goal
Adhering to these precautions will ensure the safety of your work and the quality of your scientific insights.
- If your primary focus is personal safety: Always wear gloves and glasses, handle electrolytes in a fume hood, and never mix acids with bases during cleaning.
- If your primary focus is equipment longevity: Handle the fragile cell body with care, avoid applying excessive voltage, and use non-abrasive cleaning tools.
- If your primary focus is data integrity: Ensure a perfect seal to prevent leaks, meticulously control experimental parameters, and maintain scrupulous cleanliness at every step.
Consistent and careful practice transforms this sensitive instrument into a reliable source of discovery.
Summary Table:
| Precaution Focus | Key Actions |
|---|---|
| Operator Safety | Wear protective gear, handle electrolytes in a fume hood, avoid mixing acids/bases during cleaning. |
| Equipment Integrity | Handle fragile cell body with care, ensure correct electrical polarity, avoid excessive voltage. |
| Data Accuracy | Ensure perfect cell seal to prevent leaks, control experimental parameters, maintain strict cleanliness. |
Maximize the performance and lifespan of your in-situ Raman electrolytic cell with KINTEK. Our specialized lab equipment and consumables are designed to support the precise needs of electrochemical research. From high-quality cells to reliable accessories, we provide the tools necessary for accurate, reproducible data. Contact our experts today to discuss how we can enhance your laboratory's capabilities and ensure your experiments are both safe and successful.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy



















