While X-ray Fluorescence (XRF) is a powerful and widely-used technique for elemental analysis, it is not without its inherent limitations. Its primary constraints involve difficulty in detecting very light elements, a shallow analysis depth that only measures the surface, and detection limits that are often insufficient for trace element analysis, particularly when sample preparation involves dilution.
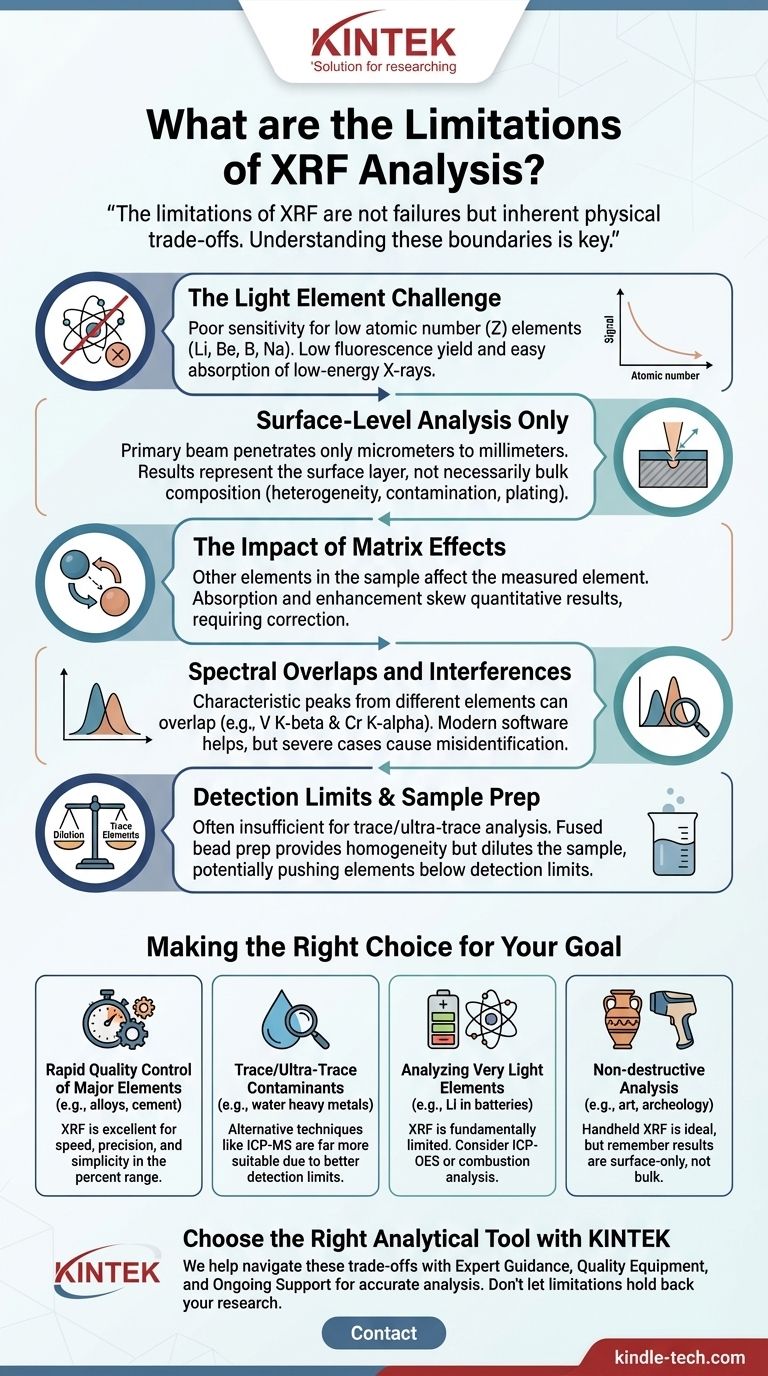
The limitations of XRF are not failures of the technology but rather inherent physical trade-offs. Understanding these boundaries—related to element weight, sample composition, and analysis depth—is the key to using XRF effectively and knowing when to choose an alternative method.

The Fundamental Constraints of XRF Analysis
To properly leverage XRF, we must first understand the physical principles that define its operational boundaries. These are not flaws but characteristics of the technique itself.
The Light Element Challenge
The primary limitation of XRF is its poor sensitivity for light elements (those with low atomic numbers, Z). Elements like lithium (Li), beryllium (Be), boron (B), and even sodium (Na) are notoriously difficult or impossible to measure.
This occurs for two main reasons. First, lighter elements have a very low fluorescence yield, meaning they are inefficient at producing characteristic X-rays. Second, the few X-rays they do produce are very low-energy and are easily absorbed by the air, the instrument's detector window, or the sample matrix itself before they can be counted.
Surface-Level Analysis Only
An XRF instrument’s primary X-ray beam only penetrates a very shallow depth into the sample, typically from a few micrometers to a few millimeters. The exact depth depends on the energy of the beam and the density of the sample material.
This means XRF is fundamentally a near-surface analysis technique. The results accurately represent the composition of the surface layer being measured but may not reflect the bulk composition of the entire object if it is heterogeneous or has surface contamination, corrosion, or plating.
The Impact of Matrix Effects
The accuracy of XRF is highly dependent on the "matrix," which is everything else in the sample besides the specific element you are trying to measure.
Other elements in the matrix can absorb the fluorescent X-rays from your element of interest (absorption) or emit X-rays that excite your element of interest further (enhancement). These matrix effects can significantly skew quantitative results if not properly corrected with sophisticated software or sample preparation methods.
Spectral Overlaps and Interferences
Each element emits a spectrum with multiple characteristic peaks (e.g., K-alpha, K-beta, L-alpha). It is common for a peak from one element to overlap with a peak from another.
For example, the K-beta peak of Vanadium (V) can overlap with the K-alpha peak of Chromium (Cr). While modern software is adept at deconvoluting these overlaps, severe cases can lead to misidentification or inaccurate quantification, especially when a trace element's peak is obscured by a major element's peak.
Understanding Sample Preparation Trade-offs
How a sample is prepared for analysis is critical and introduces its own set of limitations. The goal is to create a homogeneous sample to mitigate matrix effects, but this often involves a compromise.
The Pressed Pellet Dilemma
A common method for powders is to press them into a solid pellet. While simple, this method is susceptible to errors from particle size effects. If coarse and fine particles are distributed unevenly, the X-ray beam may disproportionately interact with one type, leading to an unrepresentative result.
The Fused Bead Compromise
To eliminate particle size issues and create a perfectly homogeneous sample, powders can be fused into a glass disk. This is done by melting the sample with a flux, such as lithium borate.
This fused bead method provides superior accuracy for major and minor elements. However, as noted in the reference, it has one major trade-off: dilution. The sample is diluted by the flux, which lowers the concentration of every element. This can easily push trace elements below the instrument's limit of detection, making them invisible to the analysis.
Making the Right Choice for Your Goal
Selecting the correct analytical method requires matching your objective to the technique's capabilities. Use these guidelines to decide if XRF is the right tool for you.
- If your primary focus is rapid quality control of major elements (e.g., alloys, cement, minerals): XRF is an excellent, industry-standard choice due to its speed, precision, and simplicity for analyzing elements in the percent range.
- If your primary focus is determining trace or ultra-trace contaminants (e.g., heavy metals in water): The inherent detection limits of XRF mean other techniques like Inductively Coupled Plasma Mass Spectrometry (ICP-MS) are far more suitable.
- If your primary focus is analyzing very light elements (e.g., lithium in batteries or carbon in steel): XRF is fundamentally limited, and you must consider alternative techniques like ICP-Optical Emission Spectrometry (ICP-OES) or combustion analysis.
- If your primary focus is non-destructive analysis of a valuable object (e.g., art, archeology): Handheld XRF is ideal, but you must interpret the results knowing you are only analyzing the immediate surface, not the bulk material.
Understanding these inherent limitations allows you to leverage XRF's strengths effectively and select the right tool for your specific analytical challenge.
Summary Table:
| Limitation | Key Constraint | Impact on Analysis |
|---|---|---|
| Light Element Detection | Poor sensitivity for low atomic number (Z) elements (e.g., Li, Be, B) | Inability to analyze key light elements in materials like batteries or steels |
| Analysis Depth | Shallow penetration (micrometers to millimeters) | Measures surface composition only; may not represent bulk material |
| Matrix Effects | Sample composition affects X-ray fluorescence (absorption, enhancement) | Can skew quantitative results without proper correction |
| Detection Limits | Insufficient for trace/ultra-trace analysis, especially after dilution | Other techniques (e.g., ICP-MS) are superior for low-concentration elements |
| Spectral Overlap | Peak interferences between elements (e.g., V K-beta and Cr K-alpha) | Potential for misidentification or inaccurate quantification |
Choose the Right Analytical Tool for Your Lab
Understanding the limitations of XRF is the first step to selecting the most effective analytical method for your specific needs. KINTEK specializes in providing laboratory equipment and consumables tailored to your unique challenges.
We help you navigate these trade-offs by offering:
- Expert Guidance: Our team can help you determine if XRF is suitable for your application or if an alternative technique (like ICP-OES or ICP-MS) is a better fit.
- Quality Equipment: From robust XRF systems to sample preparation tools for pressed pellets or fused beads, we supply reliable solutions for accurate and efficient analysis.
- Ongoing Support: We ensure your lab operates at peak performance with comprehensive service and consumables.
Don't let analytical limitations hold back your research or quality control. Contact our experts today to discuss your laboratory requirements and find the perfect solution for your elemental analysis needs.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
People Also Ask
- What does a layered film mean? Unpacking the Depths of Cinematic Storytelling
- How to measure optical properties of thin films? Master Spectroscopic Ellipsometry for Precise Results
- What temperature is maintained in a bacterial incubator? The 37°C Standard Explained
- What is the product yield of pyrolysis? Control Your Output for Biochar, Bio-oil, or Syngas
- What are the three main components of biomass? Unlocking the Secrets of Cellulose, Hemicellulose, and Lignin
- What are the risks of biomass boilers? Understanding the Environmental and Operational Trade-offs
- What is the use of pyrolysis fuel? A Sustainable Substitute for Industrial Heating and Power
- What is sputtering in manufacturing? A Guide to Precision Thin-Film Deposition



















