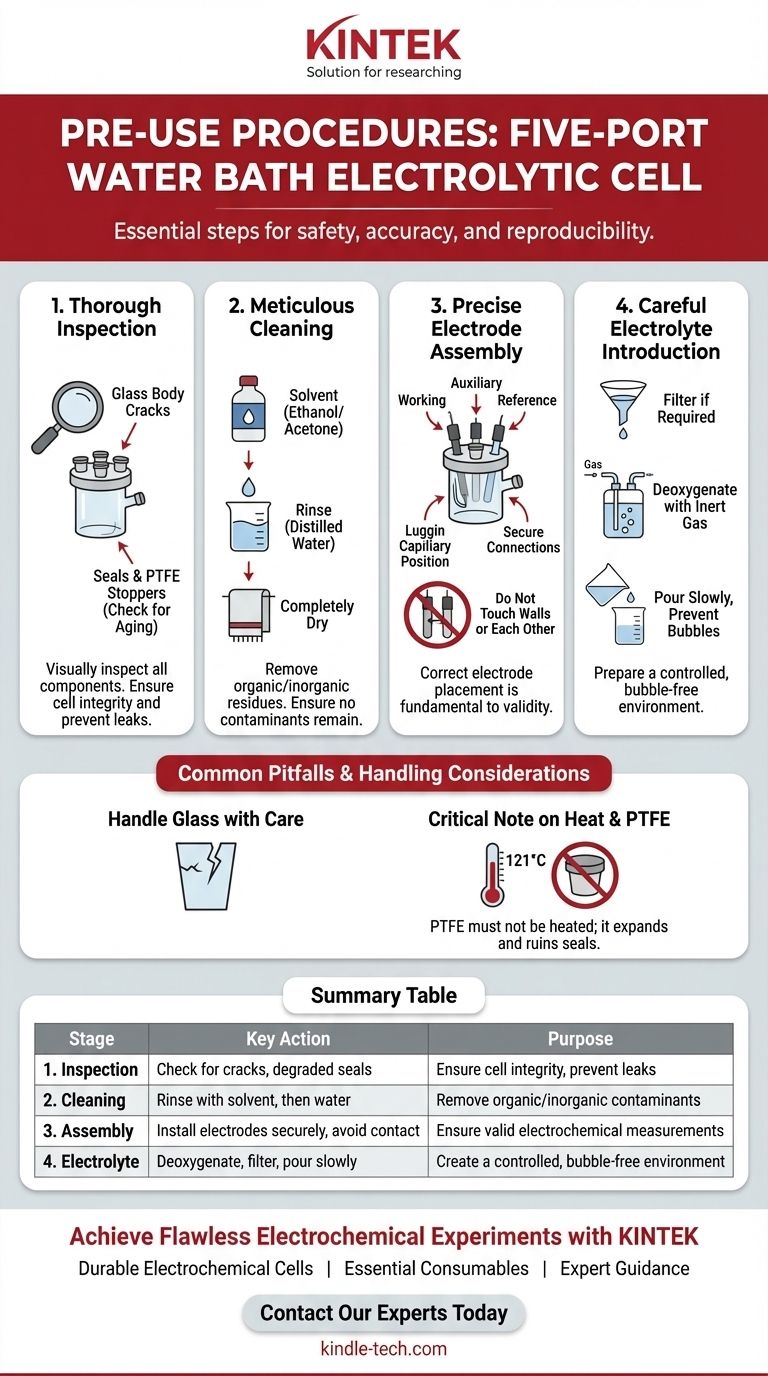
Before first use, the standard procedure for a five-port water bath electrolytic cell involves four critical stages: a thorough physical inspection of all components, meticulous cleaning to remove contaminants, precise assembly of the electrodes, and careful preparation and introduction of the electrolyte solution. Each step is essential for ensuring the safety, accuracy, and reproducibility of your electrochemical experiment.
The core principle behind these pre-use procedures is not just about setup, but about establishing a controlled and uncontaminated environment. Meticulous preparation is the foundation upon which reliable electrochemical data is built.

The Four-Stage Preparation Protocol
A systematic approach to cell preparation eliminates common sources of experimental error before your analysis even begins. Following these four stages in order is critical for consistent results.
Stage 1: Thorough Inspection
Before any other step, visually inspect every component of the cell assembly.
Look for fine cracks in the glass body, which can compromise the cell's integrity and lead to leaks. Check all seals and PTFE stoppers for signs of aging, brittleness, or degradation, as these can introduce contaminants or cause leaks.
Stage 2: Meticulous Cleaning
The goal of cleaning is to remove any organic or inorganic residues from previous experiments or from manufacturing and storage.
First, clean the cell body with a suitable organic solvent like ethanol or acetone. Follow this with a thorough rinse using distilled or deionized water to remove the solvent. Finally, ensure the cell is completely dry before proceeding with assembly.
Stage 3: Precise Electrode Assembly
Proper electrode placement is fundamental to the validity of your electrochemical measurements.
Install the working, auxiliary, and reference electrodes into the appropriate ports according to your experimental design. Ensure all connections are secure and that the electrodes are positioned correctly—critically, they should not touch the cell walls or each other. If using a Luggin capillary with your reference electrode, position its tip close to the working electrode to minimize iR drop.
Stage 4: Careful Electrolyte Introduction
The final step is preparing and adding your solution.
If required by your experiment, filter the electrolyte to remove any particulate matter. For many electrochemical systems, it is crucial to perform deoxygenation by bubbling an inert gas (like nitrogen or argon) through the solution, often using an F-type aeration tube.
Finally, pour the prepared electrolyte slowly into the cell. This helps prevent splashing and avoids the formation of bubbles on the electrode surfaces, which can block active sites and invalidate your results.
Common Pitfalls and Handling Considerations
Understanding the physical limitations of the equipment is just as important as knowing the setup procedure. Missteps here can damage the cell or ruin an experiment.
Handle with Care: The Fragility of Glass
The cell body is made of glass and is inherently fragile. Always handle it gently to prevent breakage, which can cause injury and loss of valuable materials.
A Critical Note on Heat and Sterilization
While the glass cell body can be sterilized by autoclaving at 121°C, the Polytetrafluoroethylene (PTFE) components, such as the lid and stoppers, must not be heated. PTFE will expand when heated and may not return to its original shape, ruining the seal and fit of the cell.
When to Seek Professional Repair
Do not attempt to fix complex issues yourself. Seek professional service if you encounter a malfunctioning water bath circulation system, damaged connection points, or any electrical faults.
Making the Right Choice for Your Goal
Tailor your preparation emphasis based on the specific demands of your experiment.
- If your primary focus is on trace analysis: The multi-stage cleaning protocol is your most critical step to prevent chemical contamination.
- If your primary focus is on oxygen-sensitive reactions: Rigorous and sustained deoxygenation of the electrolyte is non-negotiable.
- If your primary focus is on precise potential measurements: The correct placement of the reference electrode and its Luggin capillary is paramount.
Ultimately, a disciplined and consistent preparation routine is your best tool for achieving high-quality electrochemical data.
Summary Table:
| Stage | Key Action | Purpose |
|---|---|---|
| 1. Inspection | Check for cracks, degraded seals | Ensure cell integrity, prevent leaks |
| 2. Cleaning | Rinse with solvent (e.g., ethanol), then water | Remove organic/inorganic contaminants |
| 3. Assembly | Install electrodes securely, avoid contact | Ensure valid electrochemical measurements |
| 4. Electrolyte | Deoxygenate, filter, pour slowly | Create a controlled, bubble-free environment |
Achieve Flawless Electrochemical Experiments with KINTEK
A meticulously prepared electrolytic cell is the foundation of accurate and reproducible data. KINTEK specializes in providing the high-quality lab equipment and consumables you need to execute these critical pre-use procedures with confidence.
We support your research by providing:
- Durable Electrochemical Cells: Designed for precise assembly and long-term reliability.
- Essential Consumables: From high-purity electrolytes to reliable seals and stoppers.
- Expert Guidance: Get advice on best practices for cell maintenance and preparation.
Ready to enhance your lab's capabilities and ensure the integrity of your electrochemical work?
Contact our experts today to find the perfect solutions for your laboratory's needs.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- How should the body of an electrolytic cell be maintained for longevity? Extend Your Equipment's Lifespan



















