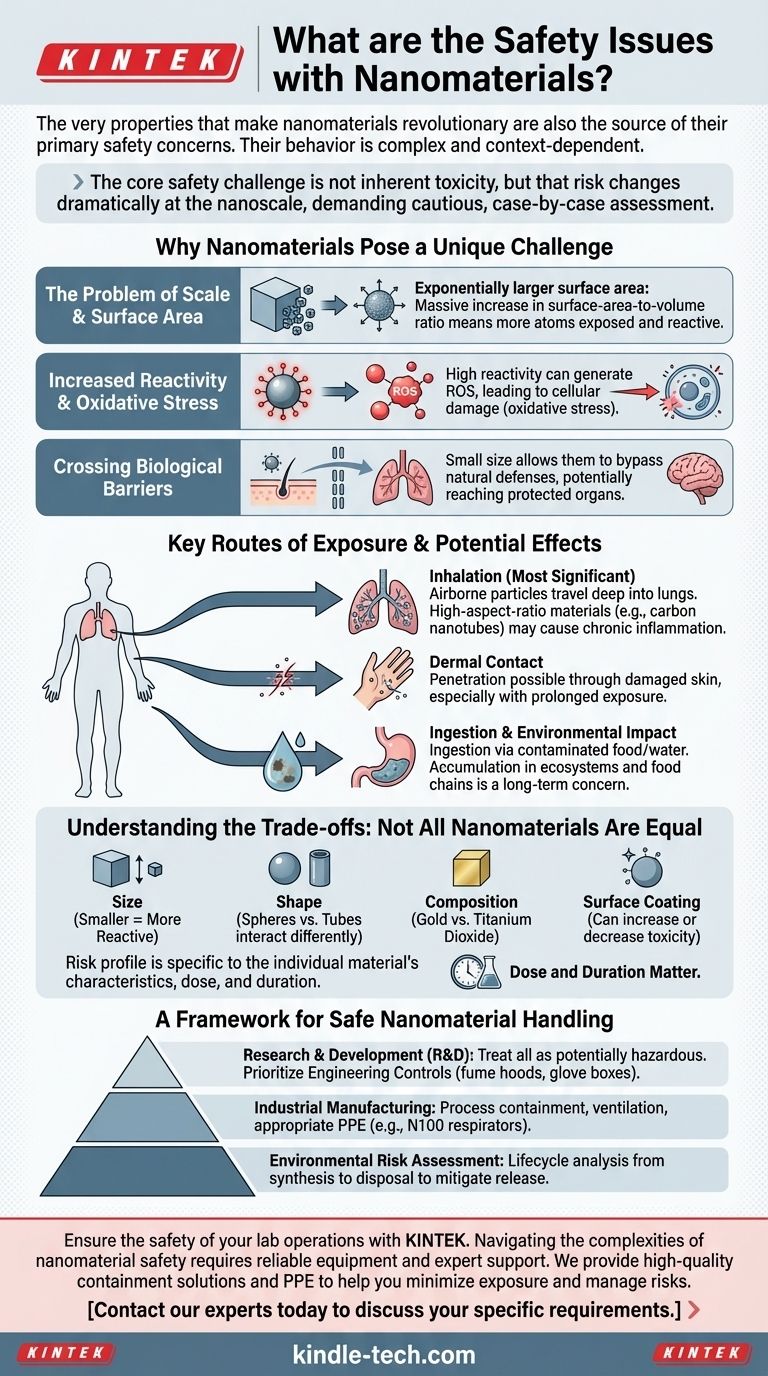
The very properties that make nanomaterials revolutionary are also the source of their primary safety concerns. Because of their incredibly small size, these materials exhibit unique physical and chemical behaviors that differ significantly from their larger, bulk counterparts. The main safety issues revolve around their high reactivity, their ability to bypass the body's natural defenses, and the current uncertainty regarding their long-term effects on human health and the environment.
The core safety challenge with nanomaterials is not that they are all inherently toxic, but that their behavior is complex and context-dependent. A material's risk profile changes dramatically at the nanoscale, demanding a cautious, case-by-case assessment based on its specific size, shape, and chemical composition.

Why Nanomaterials Pose a Unique Challenge
The transition from a bulk material to a nanomaterial is not just a change in size; it's a fundamental shift in physical and chemical properties. This shift is the origin of both their utility and their potential risk.
The Problem of Scale and Surface Area
At the nanoscale (typically 1-100 nanometers), a particle's surface-area-to-volume ratio increases exponentially. This massive surface area means a much larger proportion of the material's atoms are exposed and available to interact with their surroundings.
This is the primary driver of their heightened reactivity compared to the exact same chemical in its bulk form.
Increased Reactivity and Oxidative Stress
This high reactivity can lead to the generation of reactive oxygen species (ROS) when nanomaterials interact with biological systems.
ROS are unstable molecules that can damage cells, proteins, and DNA, a process known as oxidative stress. This cellular-level damage is a suspected mechanism behind many of the potential adverse health effects.
Crossing Biological Barriers
The small size of nanomaterials allows them to potentially bypass the body’s natural protective barriers that are effective against larger particles.
They can be inhaled deep into the lungs, may penetrate the skin, and in some cases, can even cross the highly selective blood-brain barrier, gaining access to organs and tissues that are normally protected.
Key Routes of Exposure and Potential Effects
Understanding how nanomaterials can enter the body is critical to assessing and mitigating risk, particularly in occupational and environmental contexts.
Inhalation
Inhalation is considered the most significant route of exposure for workers in nanotechnology industries. Airborne nanoparticles can travel deep into the pulmonary region.
The concern is that certain types of nanoparticles, particularly high-aspect-ratio materials like carbon nanotubes, could behave like asbestos fibers, potentially leading to chronic inflammation, fibrosis, and other lung diseases.
Dermal Contact
While intact skin provides a robust barrier, some studies suggest that very small nanoparticles might be able to penetrate it, especially if the skin is damaged, flexed, or abraded.
The overall risk from dermal contact is generally considered lower than inhalation but cannot be dismissed, especially with prolonged or high-concentration exposure.
Ingestion and Environmental Impact
Nanomaterials can be ingested through contaminated water, food, or from consumer products and medical applications. While the gastrointestinal tract presents a strong barrier, the potential for absorption and translocation to other organs exists.
When released into the environment, nanomaterials can accumulate in soil and water. Their long-term impact on ecosystems, microorganisms, and the food chain is an area of active and critical research.
Understanding the Trade-offs: Not All Nanomaterials Are Equal
It is a critical mistake to treat "nanomaterials" as a single, uniform class of substances. Their potential hazard is not a universal property but is highly specific to the individual material.
The Importance of Characterization
The risk profile of a nanoparticle is defined by a combination of factors:
- Size: Smaller particles often show greater reactivity.
- Shape: Spheres, tubes, and sheets interact with cells differently.
- Composition: A gold nanoparticle is fundamentally different from a titanium dioxide nanoparticle.
- Surface Coating: Functional coatings can either increase or decrease toxicity.
Dose and Duration Matter
Classic toxicology principles apply. The potential for harm depends on the dose (how much) and the duration of exposure (how long).
Low-level, incidental exposure to a consumer product presents a very different risk profile than chronic, high-concentration exposure in a manufacturing facility.
The Challenge of Regulation
A significant hurdle for regulators and safety professionals is the lack of standardized, globally accepted methods for detecting, measuring, and characterizing nanomaterials in complex environments like air, water, or biological tissues. This uncertainty complicates the development of clear exposure limits and safety regulations.
A Framework for Safe Nanomaterial Handling
Navigating the uncertainty surrounding nanomaterials requires a proactive and precautionary approach focused on minimizing exposure until the risks are better understood.
- If your primary focus is research and development: Treat all novel nanomaterials as potentially hazardous and implement a hierarchy of controls, prioritizing engineering solutions like fume hoods or glove boxes.
- If your primary focus is industrial manufacturing: Prioritize process containment and ventilation systems to minimize the generation and release of airborne nanoparticles, and provide appropriate personal protective equipment (PPE) like N100 respirators.
- If your primary focus is environmental risk assessment: Concentrate on the material's entire lifecycle, from synthesis to end-of-life disposal, to identify and mitigate potential release pathways into the environment.
Ultimately, harnessing the power of nanotechnology responsibly depends on a rigorous, evidence-based approach to understanding and managing its risks.
Summary Table:
| Safety Concern | Key Factor | Potential Impact |
|---|---|---|
| High Reactivity | Increased surface area | Oxidative stress, cellular damage |
| Biological Entry | Small size, ability to bypass barriers | Access to protected organs, inflammation |
| Inhalation Risk | Deep lung penetration, fiber-like behavior | Lung disease, fibrosis |
| Environmental Impact | Persistence in ecosystems | Unknown long-term effects on food chain |
| Regulatory Uncertainty | Lack of standardized detection methods | Challenges in setting exposure limits |
Ensure the safety of your lab operations with KINTEK.
Navigating the complexities of nanomaterial safety requires reliable equipment and expert support. KINTEK specializes in providing high-quality lab equipment and consumables tailored to the unique needs of nanotechnology research and development. From containment solutions like fume hoods to personal protective equipment, we help you implement the necessary controls to minimize exposure and manage risks effectively.
Let us equip your lab for safe and responsible innovation. Contact our experts today to discuss your specific requirements and how we can support your commitment to safety.
Visual Guide
Related Products
- Lab-Scale Vacuum Induction Melting Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the primary function of a vacuum induction melting furnace? Melt High-Purity Metals with Precision
- What is vacuum arc melting technique? Discover the Precision of Vacuum Induction Melting
- What is the vacuum induction method? Master High-Purity Metal Melting for Advanced Alloys
- What types of metals are typically processed in a vacuum induction melting furnace? High-Purity Alloys for Critical Applications
- How does induction work in a vacuum? Achieve Ultra-Pure Metal Melting with VIM