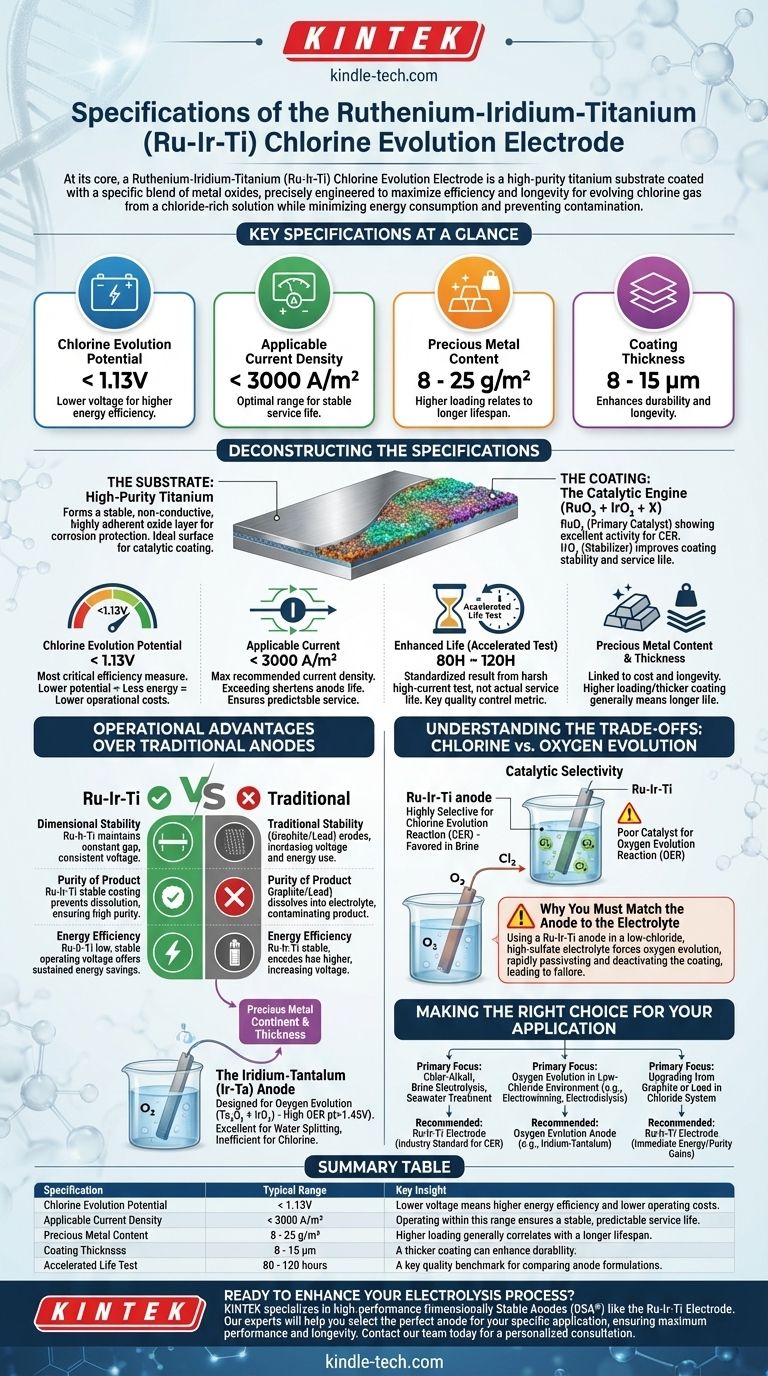
At its core, a Ruthenium-Iridium-Titanium (Ru-Ir-Ti) Chlorine Evolution Electrode is a high-purity titanium substrate coated with a specific blend of metal oxides. Its key specifications include a chlorine evolution potential below 1.13V, an applicable current density under 3000A/m², and a precious metal content of 8 to 25 g/m². The coating thickness typically ranges from 8 to 15μm, with an accelerated life rating of 80 to 120 hours.
This electrode is not just a component; it is a highly specialized catalyst. Its specifications are precisely engineered to maximize efficiency and longevity for one primary task: evolving chlorine gas from a chloride-rich solution while minimizing energy consumption and preventing contamination.
Deconstructing the Specifications: What Each Value Means
Understanding the datasheet for a mixed metal oxide (MMO) anode like this one requires knowing what each specification means for performance and durability.
The Substrate: High-Purity Titanium
The foundation of the electrode is a high-purity titanium plate, mesh, tube, or rod. Titanium is chosen because it naturally forms a stable, non-conductive, and highly adherent oxide layer (passivation) that protects the bulk metal from the corrosive electrolyte.
This passive layer serves as the ideal surface for applying the catalytically active coating.
The Coating: The Catalytic Engine (RuO₂ + IrO₂ + X)
The "magic" happens in the coating, a blend of Ruthenium Oxide (RuO₂) and Iridium Oxide (IrO₂) with other proprietary stabilizers (X).
RuO₂ is the primary catalyst, showing excellent activity for the Chlorine Evolution Reaction (CER). IrO₂ is added to improve the coating's stability and service life, preventing premature degradation.
Chlorine Evolution Potential: < 1.13V
This is the most critical measure of the anode's efficiency. It represents the electrical potential (voltage) required to drive the chlorine evolution reaction.
A lower potential is better, as it signifies that less energy is needed to produce a given amount of chlorine. This directly translates to lower operational electricity costs.
Applicable Current: < 3000A/m²
This value defines the maximum recommended operational current density. Exceeding this limit can dramatically shorten the anode's life by accelerating the wear of the catalytic coating.
Operating within this range ensures a predictable and stable service life.
Enhanced Life: 80H ~ 120H
This is not the electrode's actual service life, but a standardized result from an accelerated life test. In this test, the anode is run at a very high current density in a harsh solution to simulate years of use in a short period.
It serves as a key quality control metric and a benchmark for comparing the relative durability of different anode formulations.
Precious Metal Content & Thickness: 825g/m² & 815μm
These two values are directly linked to the anode's cost and longevity. A higher precious metal loading or a thicker coating generally results in a longer service life but also a higher initial investment.
The optimal loading depends on the specific application's required lifespan and current density.
The Operational Advantages Over Traditional Anodes
Ru-Ir-Ti anodes, as a type of Dimensionally Stable Anode (DSA®), were developed to overcome the significant drawbacks of older technologies like graphite and lead.
Dimensional Stability
Graphite anodes physically erode during electrolysis. This changes the distance between the anode and cathode, increasing cell voltage and energy consumption over time.
Ru-Ir-Ti anodes are dimensionally stable, maintaining a constant electrode gap for consistent, low-voltage operation throughout their life.
Purity of Product
Graphite and lead anodes dissolve into the electrolyte, contaminating the final product (e.g., sodium hydroxide in the chlor-alkali process).
The stable oxide coating of a Ru-Ir-Ti anode prevents this dissolution, ensuring high product purity.
Energy Efficiency
The low, stable operating voltage of these anodes offers significant and sustained energy savings compared to the higher and constantly increasing voltage of traditional anodes.
Understanding the Trade-offs: Chlorine vs. Oxygen Evolution
The most critical mistake is to assume any MMO anode will work for any process. The coating's formulation creates catalytic selectivity for a specific chemical reaction.
The Role of Coating Selectivity
The RuO₂ in a Ru-Ir-Ti anode is highly selective for the Chlorine Evolution Reaction (CER). It lowers the energy barrier for chlorine production, making it the favored reaction in a brine solution.
It is, however, a poor catalyst for the competing Oxygen Evolution Reaction (OER).
A Comparison: The Iridium-Tantalum Anode
Consider an Iridium-Tantalum (Ir-Ta) anode, which is designed for oxygen evolution. It has a different coating (Ta₂O₅ + IrO₂) and a much higher oxygen evolution potential (>1.45V).
This anode excels in processes like copper foil production or water splitting where oxygen evolution is the desired reaction, but it would be inefficient for chlorine production.
Why You Must Match the Anode to the Electrolyte
Using a Ru-Ir-Ti anode in a low-chloride, high-sulfate electrolyte is a common failure mode. In the absence of sufficient chloride ions, the anode is forced to evolve oxygen.
Because its coating is not optimized for OER, it will rapidly passivate and deactivate, leading to a spike in voltage and permanent failure.
Making the Right Choice for Your Application
Your choice of anode must be dictated by the chemistry of your electrolytic process.
- If your primary focus is chlor-alkali, brine electrolysis, or seawater treatment: The Ru-Ir-Ti electrode is the industry standard due to its high selectivity and efficiency for chlorine evolution.
- If your primary focus is oxygen evolution in a low-chloride environment (e.g., electrowinning from sulfate solutions, electrodialysis): You must select an oxygen evolution anode, such as an Iridium-Tantalum (Ir-Ta) model, as the Ru-Ir-Ti anode will fail prematurely.
- If your primary focus is upgrading from graphite or lead anodes in a chloride system: The Ru-Ir-Ti electrode offers significant, immediate gains in energy efficiency, product purity, and operational stability.
Ultimately, selecting the correct anode is about matching the catalyst to the specific chemical reaction you intend to drive.
Summary Table:
| Specification | Typical Range | Key Insight |
|---|---|---|
| Chlorine Evolution Potential | < 1.13V | Lower voltage means higher energy efficiency and lower operating costs. |
| Applicable Current Density | < 3000 A/m² | Operating within this range ensures a stable, predictable service life. |
| Precious Metal Content | 8 - 25 g/m² | Higher loading generally correlates with a longer lifespan. |
| Coating Thickness | 8 - 15 μm | A thicker coating can enhance durability. |
| Accelerated Life Test | 80 - 120 hours | A key quality benchmark for comparing anode formulations. |
Ready to enhance your electrolysis process with the right electrode?
KINTEK specializes in high-performance lab equipment and consumables, including a full range of Dimensionally Stable Anodes (DSA®) like the Ru-Ir-Ti Chlorine Evolution Electrode. We help laboratories and industrial facilities achieve superior efficiency, product purity, and operational stability.
Our experts will help you select the perfect anode for your specific application, ensuring maximum performance and longevity.
Contact our team today for a personalized consultation and discover how KINTEK can power your innovations.
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Aluminum Foil Current Collector for Lithium Battery
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
People Also Ask
- What is a common use for a platinum sheet electrode? As a Reliable Counter Electrode in Electrochemical Cells
- What can cause poisoning of a platinum disk electrode and how can it be prevented? Ensure Reliable Electrochemical Data
- What are the standard specifications for platinum wire and rod electrodes? Select the Right Form Factor for Your Experiment
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data
- What are platinum electrodes used for? Essential Uses in Science, Medicine, and Industry















