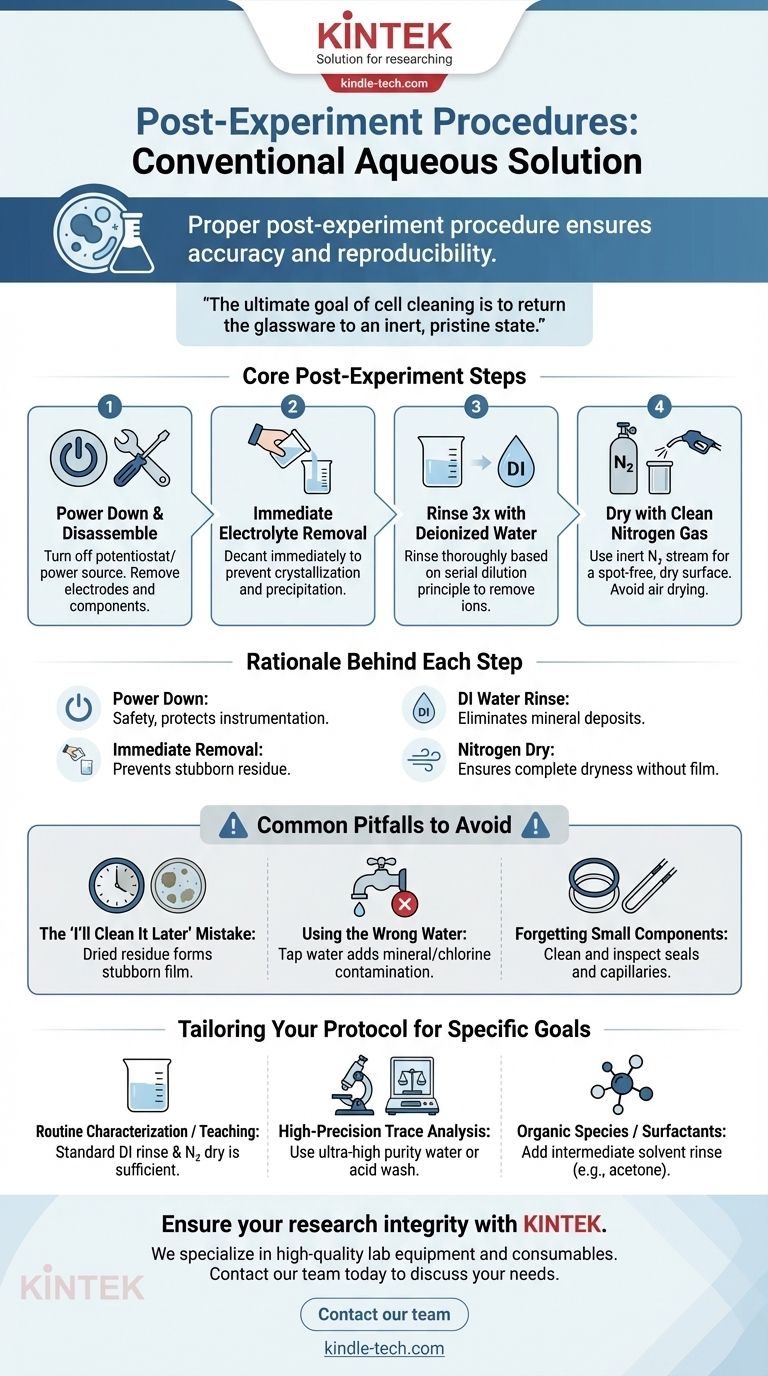
Proper post-experiment procedure is not merely about tidiness; it is a fundamental step that ensures the accuracy and reproducibility of your future work. After using a cell with a conventional aqueous solution, you must power down the equipment, remove the electrodes, pour out the electrolyte, immediately rinse the cell at least three times with deionized water, and finally dry it with a clean nitrogen stream.
The ultimate goal of cell cleaning is to return the glassware to an inert, pristine state. Any residual electrolyte or contamination left behind becomes an uncontrolled variable in your next experiment, directly compromising the integrity of your results.

The Rationale Behind Each Cleaning Step
A disciplined cleaning protocol is built on a clear understanding of why each step is performed. It moves beyond a simple checklist to become a critical part of the scientific method itself.
Step 1: Power Down and Disassemble
Before any handling, ensure the potentiostat or power source is turned off. This fundamental safety measure prevents electrical shock and protects the sensitive instrumentation from damage during disassembly. Only then should you carefully remove the electrodes and any other components from the cell.
Step 2: Immediate Removal of the Electrolyte
The electrolyte must be decanted immediately after the experiment concludes. As the solution evaporates, the concentration of salts increases, leading to crystallization and precipitation on the cell walls and electrode surfaces. These dried residues can be difficult to remove and can permanently alter the surface of your cell.
Step 3: The Critical Deionized Water Rinse
The cell must be rinsed thoroughly, and deionized (DI) water is the correct tool for the job. Unlike tap water, DI water contains no dissolved ions, so it will not leave behind mineral deposits (e.g., calcium carbonate) as it dries.
Rinsing three times is standard practice based on the principle of serial dilution. Each rinse dramatically reduces the concentration of the remaining contaminants, effectively washing them away and leaving a clean surface.
Step 4: The Final Nitrogen Dry
Drying with a gentle stream of clean nitrogen gas is the final step. Air drying is slow and can leave "water spots" from any remaining trace impurities. Furthermore, compressed air from a house line often contains oil and particulate matter.
Nitrogen, being dry and inert, effectively displaces water droplets without reacting with the cell surface or leaving a film. This ensures the cell is not only clean but also completely dry and ready for storage or immediate reuse.
Common Pitfalls to Avoid
Even with a defined procedure, small mistakes can lead to significant experimental error down the line. Awareness of these common pitfalls is crucial for maintaining high-quality data.
The "I'll Clean It Later" Mistake
The single most common error is delaying the cleaning process. Once electrolyte residue dries and solidifies, it often forms a stubborn film that will not dissolve easily in DI water. Removing it may require aggressive methods like sonication or washing with acids, which can risk damaging the cell.
Using the Wrong Water
Rinsing with tap water is a critical error. The dissolved minerals and chlorine present in most tap water will adsorb onto the cell's interior surface, creating a layer of contamination that will interfere with future electrochemical measurements.
Forgetting the Small Components
Contamination often hides in the details. Remember to clean and inspect all O-rings, seals, and Luggin capillaries. These components can trap electrolyte and should be rinsed and dried with the same care as the main cell body.
Making the Right Choice for Your Goal
Your cleaning protocol should be as rigorous as your experimental needs. While the standard procedure is a robust baseline, certain goals demand higher levels of care.
- If your primary focus is routine characterization or teaching: The standard procedure of immediate DI water rinsing and nitrogen drying is sufficient for achieving consistent and reliable results.
- If your primary focus is high-precision trace analysis: You must consider a more stringent protocol, which may involve a final rinse with ultra-high purity water or even a dilute acid wash (e.g., with nitric acid) followed by extensive DI water rinsing to remove any adsorbed metal ions.
- If you have used solutions with organic species or surfactants: You may need an intermediate rinse with a solvent like acetone or isopropanol (after the DI water rinse) to remove organic residues before the final drying step.
By treating cell cleaning as an integral part of the experiment itself, you ensure the reliability and quality of your future research.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1 | Power Down & Disassemble | Ensure safety and protect instrumentation. |
| 2 | Immediate Electrolyte Removal | Prevent salt crystallization and surface damage. |
| 3 | Rinse 3x with Deionized Water | Remove ionic contaminants via serial dilution. |
| 4 | Dry with Clean Nitrogen Gas | Achieve a spot-free, inert, and dry surface. |
Ensure your research integrity with the right equipment and protocols. Proper post-experiment care is essential for reliable results. KINTEK specializes in high-quality lab equipment and consumables for electrochemical and analytical laboratories. Let our experts help you select the right cells, electrodes, and cleaning supplies to maintain pristine labware and achieve consistent, high-quality data.
Contact our team today to discuss your specific laboratory needs and optimize your workflow.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy



















