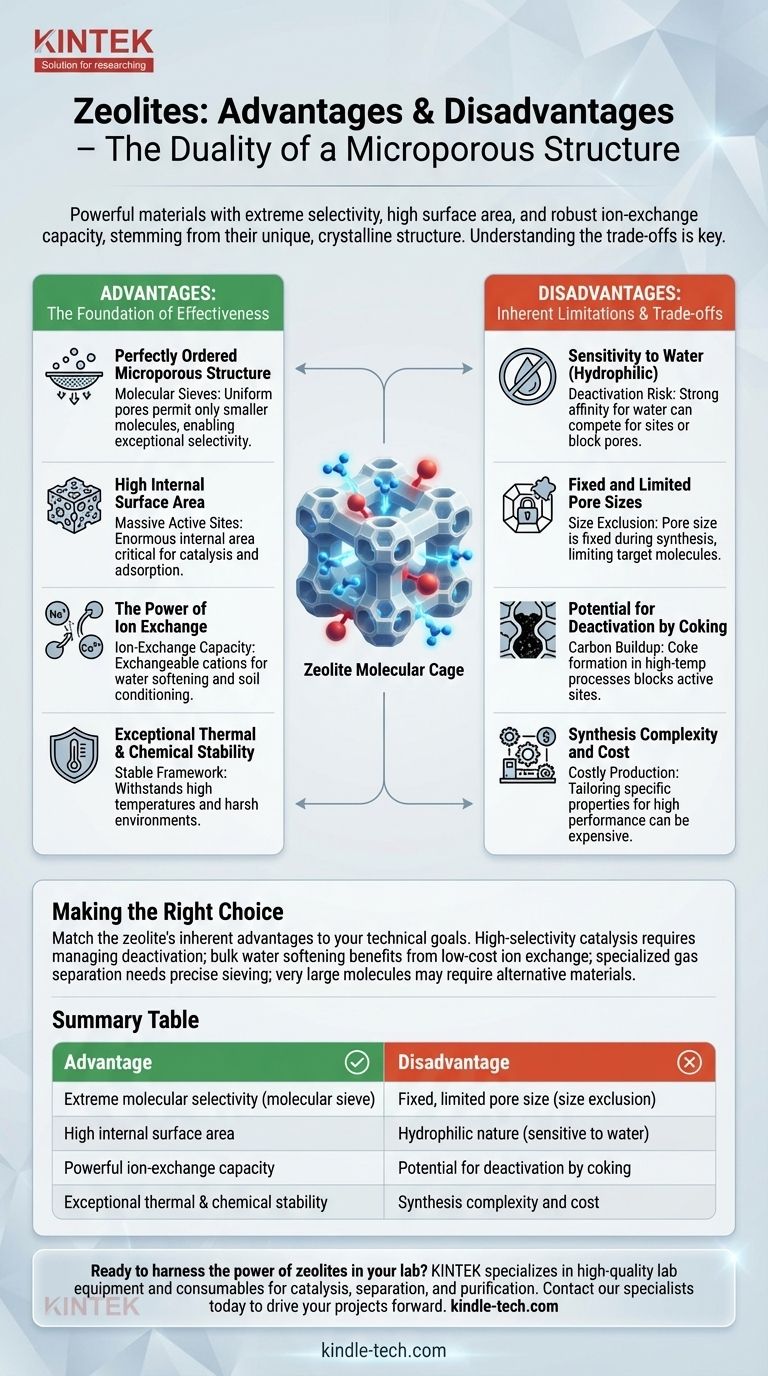
At their core, zeolites are powerful materials whose primary advantages—extreme selectivity, high surface area, and robust ion-exchange capacity—stem directly from their unique, crystalline microporous structure. Their main disadvantages, such as a fixed pore size and potential deactivation by water, are the unavoidable flip side of these same structural properties. Understanding this duality is the key to deploying them effectively.
The immense utility of zeolites comes from their rigid, perfectly uniform "molecular cages." This structure makes them unparalleled for selectively filtering, exchanging, or catalyzing specific molecules, but it also defines their limitations, as these cages can become blocked or are simply the wrong size for a given task.

The Foundation: Why Zeolites Are So Effective
The advantages of zeolites are not a random collection of features; they all derive from the material's fundamental atomic arrangement.
A Perfectly Ordered Microporous Structure
Zeolites are crystalline aluminosilicates with a framework of interconnected cavities, or pores. Unlike amorphous materials like activated carbon, these pores are of a precise and uniform size.
This uniformity allows them to act as molecular sieves, only permitting molecules smaller than their pore openings to enter. This is the basis of their exceptional selectivity.
High Internal Surface Area
While a zeolite crystal appears to be a solid particle, its internal network of pores creates an enormous surface area. A single gram of zeolite can have a surface area of several hundred square meters.
This massive internal area is critical for applications like catalysis and adsorption, as it provides a vast number of active sites for chemical reactions or for capturing target molecules.
The Power of Ion Exchange
The zeolite framework has a net negative charge, which is balanced by mobile, positively charged ions (cations like Na⁺ or Ca²⁺) residing within the pores.
These cations are not permanently fixed and can be easily swapped with other cations from a surrounding solution. This ion-exchange capacity is the fundamental principle behind their use in water softening and as soil conditioners.
Exceptional Thermal and Chemical Stability
The framework of a zeolite is built from strong silicon-oxygen and aluminum-oxygen bonds. This makes the crystal structure remarkably stable, allowing it to withstand high temperatures and harsh chemical environments often found in industrial processes.
Understanding the Inherent Disadvantages
The very properties that make zeolites advantageous also create their limitations. These are not flaws, but trade-offs that must be managed.
Sensitivity to Water (Hydrophilic Nature)
Most common and inexpensive zeolites are hydrophilic, meaning they have a strong affinity for water molecules.
In many catalytic or gas separation processes, water can compete for active sites or physically block the pores, effectively deactivating the zeolite. While hydrophobic zeolites exist, they are often more complex and costly to synthesize.
Fixed and Limited Pore Sizes
The "molecular sieve" effect is also a major constraint. The pore size of a given zeolite is fixed during its synthesis.
If your target molecule is larger than the zeolite's pores, it simply cannot enter the crystal to react or be adsorbed. This size exclusion means you must choose or synthesize a zeolite with a pore system that precisely matches your application.
Potential for Deactivation by Coking
In high-temperature hydrocarbon catalysis, carbonaceous deposits, known as coke, can form inside the zeolite pores.
This coke physically blocks access to the active sites, leading to a gradual loss of activity. While the zeolite can often be regenerated by burning off the coke, this requires process downtime and adds operational complexity.
Synthesis Complexity and Cost
While many types of zeolites occur naturally and are inexpensive, they often have impurities and variable properties.
Synthesizing a specific zeolite with a desired framework, pore size, and Si/Al ratio for a high-performance application can be a complex and costly process. It may require high pressure, high temperature, and expensive template molecules to guide the crystal's formation.
Making the Right Choice for Your Application
The decision to use a zeolite depends entirely on whether its inherent advantages align with your primary technical and economic goals.
- If your primary focus is high-selectivity catalysis: The shape-selective nature of zeolites is unparalleled, but you must engineer a process that manages deactivation from water or coking.
- If your primary focus is bulk water softening or purification: The powerful ion-exchange capability of inexpensive, natural zeolites makes them an excellent and highly economical choice.
- If your primary focus is specialized gas separation: The precise molecular sieving of synthetic zeolites offers superior performance, but you must ensure the value of the separated product justifies the material's cost.
- If you are working with very large molecules: The microporosity of zeolites is a fundamental limitation; you should investigate alternative materials like mesoporous silicas.
Ultimately, harnessing the power of zeolites requires matching your specific molecular challenge to the unique structural properties of the right zeolite framework.
Summary Table:
| Advantage | Disadvantage |
|---|---|
| Extreme molecular selectivity (molecular sieve) | Fixed, limited pore size (size exclusion) |
| High internal surface area for catalysis/adsorption | Hydrophilic nature (sensitive to water, can cause deactivation) |
| Powerful ion-exchange capacity | Potential for deactivation by coking in high-temperature processes |
| Exceptional thermal and chemical stability | Synthesis complexity and cost for specific, high-performance types |
Ready to harness the power of zeolites in your lab?
KINTEK specializes in providing high-quality lab equipment and consumables, including materials tailored for catalysis, separation, and purification processes. Our experts can help you select the right materials to overcome challenges like pore size limitations or deactivation, ensuring your research or production is efficient and effective.
Contact our specialists today to discuss how we can support your specific laboratory needs and drive your projects forward.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory Vibratory Sieve Shaker Machine Slap Vibrating Sieve
- Single Punch Manual Tablet Press Machine TDP Tablet Punching Machine
People Also Ask
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- Why is PTFE wire used for hanging metal specimens in biodiesel corrosion tests? Ensure Pure Experimental Results
- What are the specific applications of PTFE in micro-batch slug flow systems? Enhance Your Microfluidic Reaction Purity
- Why are PTFE laboratory consumables required when testing stainless steel against organic acids? Ensure Data Integrity
- How are PTFE gaskets utilized for POEGMA electrolyte conductivity? Ensure Precision in Electrochemical Measurements



















