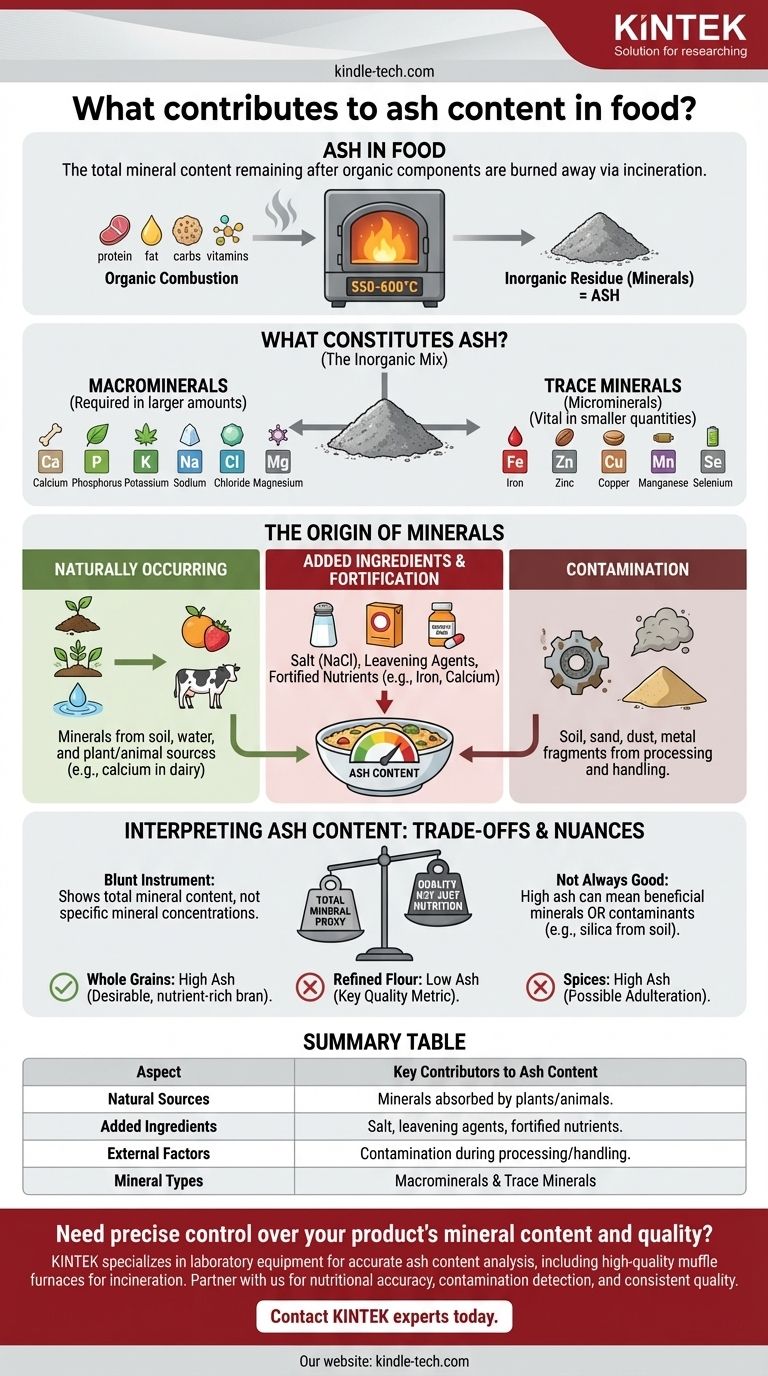
In short, ash content in food represents the total amount of minerals, or the inorganic residue, that remains after the organic components like protein, fat, and carbohydrates are completely burned away. This measurement is a fundamental proxy for the total mineral content of a food product.
The term "ash" can be misleading. It doesn't refer to the byproduct of a simple fire, but rather the result of a precise laboratory process called incineration. This process reveals a food's mineral footprint, which is a critical indicator of its nutritional value, purity, and processing history.

What Constitutes "Ash"? A Deeper Look
The ash value on a specification sheet is a single number, but it represents a complex mixture of essential and non-essential inorganic elements. Understanding this composition is key to its proper interpretation.
From Organic to Inorganic
To measure ash, a food sample is heated to a very high temperature (typically 550-600°C) in a specialized furnace. This process, known as incineration or dry ashing, systematically burns off all organic matter—water, fats, proteins, carbohydrates, and vitamins—leaving only the non-combustible inorganic minerals behind.
The Minerals That Remain
The resulting ash is a collection of all the minerals present in the original food. These can be broadly categorized into two groups.
- Macrominerals: These are required by the body in larger amounts and are often the largest contributors to ash content. They include calcium, phosphorus, potassium, sodium, chloride, and magnesium.
- Trace Minerals (Microminerals): These are needed in smaller quantities but are still vital for health. This group includes iron, zinc, copper, manganese, and selenium, among others.
The Origin of Minerals in Food
The mineral content, and therefore the ash content, of a food is not static. It is determined by the food's source, how it was grown, and how it was processed.
Naturally Occurring Minerals
The primary source of ash is the natural mineral content of the raw ingredients. Plants absorb minerals directly from the soil and water they grow in, while animals accumulate minerals from the plants and water they consume. For example, dairy products have a high ash content due to their significant natural calcium levels.
Added Ingredients and Fortification
Many processed foods contain ingredients that are themselves minerals or have minerals added for functional or nutritional reasons. Common examples include:
- Salt (Sodium Chloride): A major contributor to ash in cured meats, snacks, and canned goods.
- Leavening Agents: Baking soda (sodium bicarbonate) or baking powder contain sodium and sometimes calcium or aluminum.
- Fortified Nutrients: Cereals are often fortified with iron, and plant-based milks may be fortified with calcium carbonate. These directly increase the final ash content.
Contamination from Processing and Handling
Ash can also include undesirable inorganic matter introduced during production. This is a critical point for quality control. Sources can include traces of soil, sand, or dust on raw produce or small metal fragments from processing machinery. A higher-than-expected ash value can be a red flag for contamination.
Understanding the Trade-offs and Nuances
While a valuable metric, total ash content has limitations. Interpreting the number requires understanding its context.
"Total Ash" is a Blunt Instrument
The standard ash test provides a single figure for total mineral content. It cannot tell you the specific amount of calcium versus the amount of sodium. To determine the concentration of individual minerals, more advanced analytical techniques like atomic absorption spectroscopy are required.
Not All Ash is Nutritionally Valuable
A high ash value is not inherently "good." While it can indicate a high concentration of beneficial minerals like calcium and iron, it can also signal the presence of nutritionally insignificant or even harmful substances. For instance, high levels of silica (from soil contamination) will increase ash content but provide no nutritional benefit.
Context Determines Quality
The "ideal" ash content is entirely dependent on the product.
- High Ash in Whole Grains: Expected and desirable, as the mineral-rich bran and germ are intact.
- Low Ash in Refined Flour: A key quality metric, indicating effective removal of bran and germ.
- High Ash in Spices: Can be a sign of adulteration with sand or soil to increase weight.
How to Interpret Ash Content for Your Goal
Your objective determines how you should interpret the ash value on a technical data sheet.
- If your primary focus is nutritional labeling: Ash content is your starting point for verifying the total mineral claim on a product's nutrition panel.
- If your primary focus is quality control: Use ash content as a rapid screening tool to detect adulteration, confirm ingredient consistency, and monitor for process contamination.
- If your primary focus is product development: Monitor ash levels to understand how different ingredients and processing steps impact the final product's mineral profile and functional properties.
Ultimately, ash content is a simple yet powerful measure that provides a window into a food's composition, quality, and authenticity.
Summary Table:
| Aspect | Key Contributors to Ash Content |
|---|---|
| Natural Sources | Minerals absorbed by plants from soil; minerals from animal diets (e.g., calcium in dairy). |
| Added Ingredients | Salt (sodium chloride), leavening agents (baking soda), fortified nutrients (iron, calcium). |
| External Factors | Contamination from soil, sand, dust, or metal fragments during processing/handling. |
| Mineral Types | Macrominerals (Calcium, Potassium) and Trace Minerals (Iron, Zinc). |
Need precise control over your product's mineral content and quality?
At KINTEK, we specialize in the laboratory equipment necessary for accurate ash content analysis, including high-quality muffle furnaces for precise incineration. Whether you are in food manufacturing, quality control, or product development, our tools help you ensure nutritional accuracy, detect contamination, and maintain consistent product quality.
Let KINTEK be your partner in achieving reliable results. Contact our experts today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of a muffle furnace in Nb-O coated NMC powder preparation? Optimize Your Material Purity
- What is the role of a high-temp furnace in alloy pre-oxidation? Enhance High-Aluminum Alloy Durability
- What is the primary function of a high-temperature muffle furnace for Ga/HZSM-5? Optimize Your Catalyst Preparation
- Why are high-temperature carbonization furnaces and activation essential for supercapacitor activated carbon?
- Why is a laboratory constant temperature drying oven required to process rice husks? Ensure Composite Quality
- What is the inside of a muffle furnace? Discover the Key Components for Precise High-Temperature Processing
- What is the purpose of the ash content test? A Guide to Material Quality Control
- What is a muffle furnace test? Achieve Precise, Contamination-Free Heating for Your Lab



















