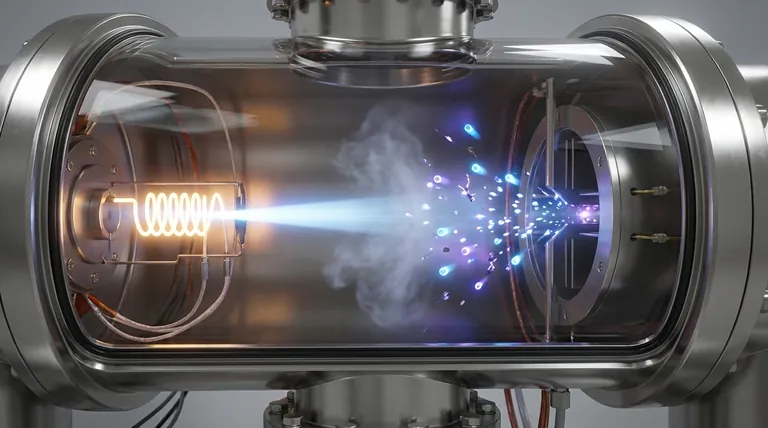
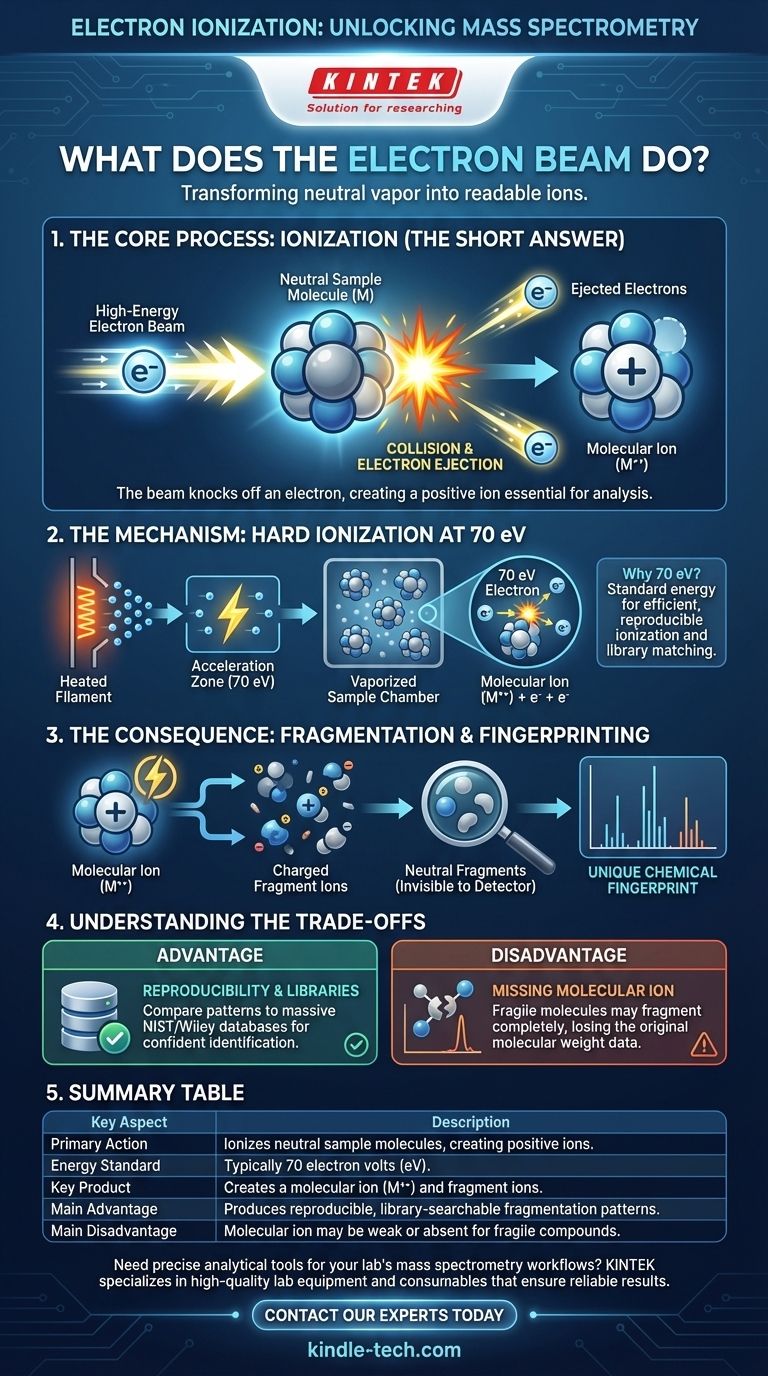
In short, the beam of electrons ionizes the sample. It collides with the neutral molecules in the vapor, knocking one of their own electrons out. This transforms the neutral molecules into positively charged ions, a crucial step that allows them to be controlled and analyzed by the mass spectrometer.
The fundamental purpose of the electron beam is to impart a positive charge onto the sample molecules. This conversion from neutral to charged is what makes mass analysis possible, as only ions can be accelerated and separated by electric and magnetic fields.
The Mechanism of Electron Ionization (EI)
The process you're asking about is a "hard" ionization technique known as Electron Ionization (EI). It's a foundational method in mass spectrometry, particularly for identifying unknown organic compounds.
The Collision Event
A heated filament, typically made of tungsten or rhenium, releases a stream of electrons. These electrons are then accelerated across a voltage gap, usually to a standard energy of 70 electron volts (70 eV). This high-energy beam is directed through the vaporized sample.
Creating the Molecular Ion
When a 70 eV electron strikes a neutral sample molecule (M), its energy is high enough to dislodge one of the molecule's own electrons.
The result is a positively charged radical cation, known as the molecular ion (M+•). The original electron and the dislodged electron are then removed from the system.
Why 70 eV is the Standard
This specific energy level is used because it's well above the energy required to ionize most organic molecules (typically 7-15 eV). This ensures efficient ionization and, critically, produces highly reproducible results that can be compared to vast spectral libraries for compound identification.
The Critical Consequence: Fragmentation
The 70 eV of energy transferred during the collision is often far more than the molecule can handle. This excess energy causes the newly formed molecular ion to break apart into smaller pieces.
A Predictable Fingerprint
This process, called fragmentation, is not random. A specific molecule will consistently break apart in the same way, producing a characteristic pattern of smaller, charged fragment ions.
This fragmentation pattern acts as a unique chemical fingerprint. By analyzing the masses of these fragments, chemists can deduce the original structure of the unknown molecule.
What the Mass Spectrometer Detects
It is crucial to understand that the mass spectrometer only detects and analyzes the charged particles. This includes the original molecular ion (if it's stable enough to survive) and the various charged fragment ions. Any neutral fragments that break off are invisible to the detector.
Understanding the Trade-offs
Like any analytical technique, Electron Ionization has distinct advantages and disadvantages that are critical to understand.
The Advantage: Reproducibility and Libraries
The primary strength of EI is its reproducibility. Because the 70 eV standard is so widely used, massive, searchable databases (like the NIST and Wiley libraries) exist. You can compare the fragmentation pattern of your unknown sample against these libraries to find a match, making it a powerful tool for identification.
The Disadvantage: The Missing Molecular Ion
The main drawback of this "hard" ionization method is that some molecules are too fragile. The molecular ion may fragment so completely that very little, or none, of it reaches the detector. When this happens, you lose the single most important piece of data: the molecular weight of the original compound.
How This Impacts Your Analysis
Understanding this process allows you to interpret your results correctly and choose the right method for your goal.
- If your primary focus is identifying a common unknown compound: The rich fragmentation pattern produced by EI is your most powerful tool for a confident library search.
- If your primary focus is determining the molecular weight of a new or fragile molecule: Be aware that the molecular ion peak may be weak or absent with EI, and a "softer" ionization technique may be necessary.
Ultimately, the electron beam transforms an invisible, neutral molecule into a readable and identifiable chemical signature.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Action | Ionizes neutral sample molecules, creating positive ions. |
| Energy Standard | Typically 70 electron volts (eV). |
| Key Product | Creates a molecular ion (M+•) and fragment ions. |
| Main Advantage | Produces reproducible, library-searchable fragmentation patterns. |
| Main Disadvantage | Molecular ion may be weak or absent for fragile compounds. |
Need precise analytical tools for your lab's mass spectrometry workflows? KINTEK specializes in high-quality lab equipment and consumables that ensure reliable results. From robust ionization sources to the consumables that keep your instruments running, we support your laboratory's needs for accurate compound identification. Contact our experts today to discuss how we can enhance your analytical capabilities!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Three-dimensional electromagnetic sieving instrument
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- What is a thin film circuit? Achieve Unmatched Miniaturization & High-Frequency Performance
- What is the function of a heating reaction system in benzoic acid esterification? Master Precision Thermal Control
- How does the working of oil-free diaphragm vacuum pumps differ from conventional pumps? A Guide to Clean vs. Deep Vacuum
- What is the role of a laboratory constant temperature drying oven in anaerobic digestion? Precision TS Analysis
- What is the primary function of a vacuum drying oven in Pyr-IHF synthesis? Ensure High-Purity Cathode Material Quality
- Can you provide a typical example of the calcination process? Discover the Limestone to Lime Transformation
- What are the possible reasons why a joint may be impossible to braze? A Guide to Overcoming Common Brazing Failures
- What is needed for annealing? Master the 3 Critical Stages for Material Transformation














