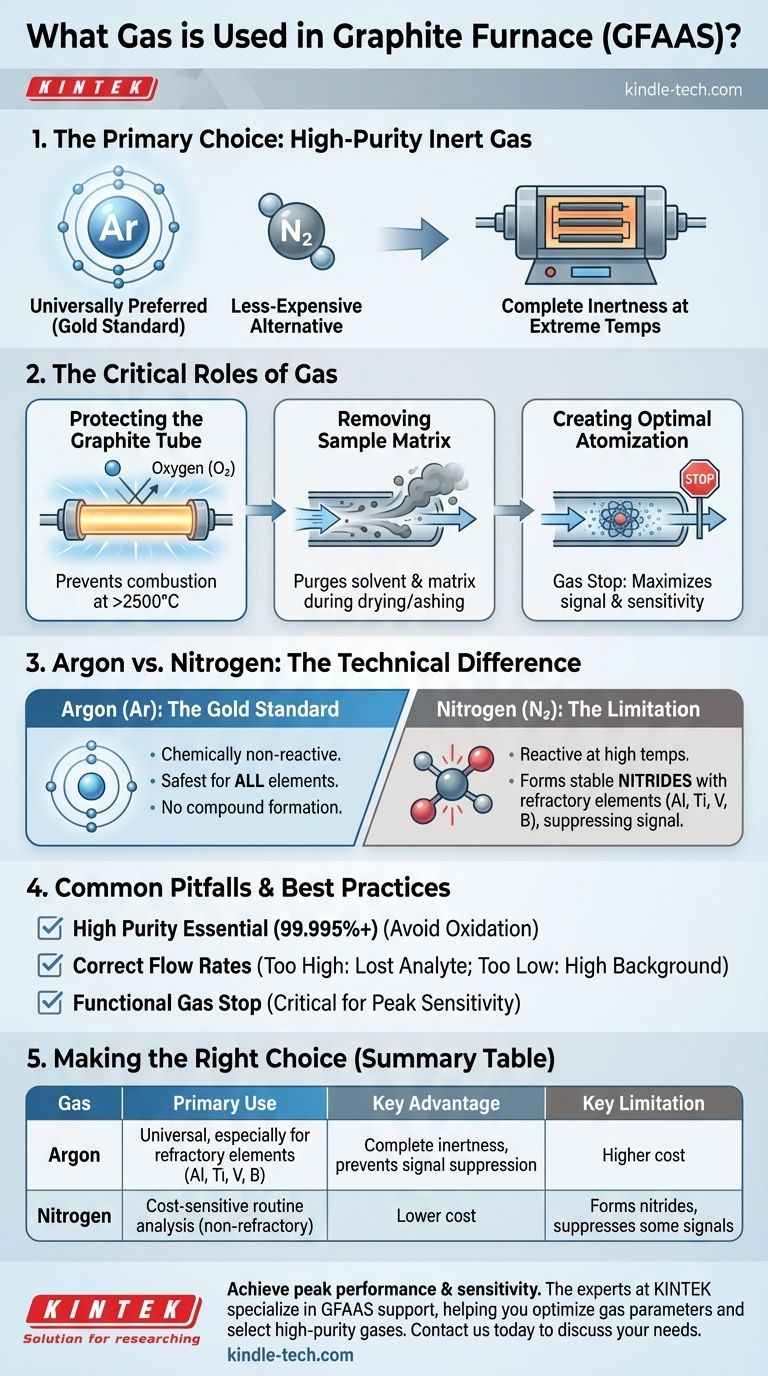
In graphite furnace atomic absorption spectroscopy (GFAAS), the standard gas used is a high-purity inert gas, most commonly argon. While nitrogen is sometimes used as a less-expensive alternative, argon is the universally preferred choice due to its complete inertness at the extreme temperatures required for analysis.
The core function of the gas in a graphite furnace is not just to be inert, but to perform two distinct, critical roles: protecting the graphite tube from incineration and actively removing the sample matrix to ensure a clean, interference-free measurement.

The Critical Role of the Inert Gas
Understanding why the gas is used is fundamental to operating the instrument correctly and achieving accurate results. The gas serves several purposes throughout the temperature program.
Protecting the Graphite Tube
The furnace heats a graphite tube to temperatures often exceeding 2500°C. At these temperatures, the carbon of the tube would instantly combust if exposed to oxygen from the air.
A continuous flow of inert gas, typically argon, provides a protective blanket around and through the tube, preventing its rapid degradation and ensuring a stable analytical environment.
Removing the Sample Matrix
During the initial heating stages (drying and pyrolysis/ashing), a controlled flow of argon is passed through the inside of the tube.
This internal gas flow acts as a physical purge, sweeping away the vaporized solvent and unwanted matrix components from the sample before the final measurement step. This is crucial for minimizing background noise and chemical interferences.
Creating an Optimal Atomization Environment
During the final, high-temperature atomization step, the internal gas flow is stopped. This is known as the "gas stop" phase.
Stopping the flow creates a static, dense cloud of analyte atoms within the graphite tube. This momentary containment maximizes the time the atoms spend in the instrument's light path, which dramatically increases the absorption signal and improves analytical sensitivity.
Argon vs. Nitrogen: The Technical Difference
While both are inert gases, their behavior at high temperatures dictates their suitability for GFAAS.
Why Argon is the Gold Standard
Argon is a noble gas, meaning it is chemically non-reactive under almost all conditions. It will not form compounds with the analyte or the graphite, even at peak atomization temperatures.
This complete inertness makes argon the safest and most reliable choice for nearly any element, ensuring that the measured signal is not suppressed by unwanted chemical reactions.
The Limitation of Nitrogen
Nitrogen is often cheaper than argon, making it an attractive alternative. For many analyses, it performs adequately.
However, at very high temperatures, nitrogen can become reactive and form stable nitrides with certain elements, particularly refractory elements like aluminum (Al), titanium (Ti), vanadium (V), and boron (B). This chemical reaction "traps" the analyte, preventing it from atomizing correctly and leading to a suppressed signal and inaccurate low readings.
Understanding the Trade-offs and Common Pitfalls
Improper gas management is a frequent source of poor performance in GFAAS.
The Critical Need for High Purity
You must use high-purity or "instrument grade" gas (typically 99.995% or higher). Impurities, especially oxygen, will significantly shorten the lifetime of your graphite tubes by causing gradual oxidation.
Even trace amounts of oxygen can create background signals or interfere with the analysis, compromising your results.
Consequences of Incorrect Flow Rates
Setting the internal gas flow too high during the pyrolysis step can cause physical expulsion of the analyte along with the matrix, leading to poor sensitivity.
Conversely, setting the flow too low may not adequately remove the matrix, resulting in high background signals that obscure the analyte measurement. Always follow the manufacturer's recommendations for your specific application.
The Impact of a Failed Gas Stop
If the gas flow does not stop properly during the atomization step, the newly formed atomic cloud will be swept out of the tube too quickly.
This results in a sharp decrease in signal intensity and a significant loss of sensitivity, making it impossible to measure low concentrations accurately.
Making the Right Choice for Your Analysis
Your choice of gas depends directly on your analytical goals and the elements you are measuring.
- If your primary focus is maximum accuracy and method versatility: Use high-purity argon. It is the universally accepted standard that eliminates the risk of signal suppression for any element.
- If your primary focus is cost reduction for routine analysis: Nitrogen can be a viable option, but you must first validate that it does not suppress the signal for your specific analytes of interest.
- If you are analyzing Al, Ti, V, B, or other refractory elements: Use argon exclusively. The risk of nitride formation with nitrogen is too high and will lead to inaccurate results.
Proper gas selection and control are fundamental to achieving the high sensitivity and reliability that define graphite furnace analysis.
Summary Table:
| Gas | Primary Use Case | Key Advantage | Key Limitation |
|---|---|---|---|
| Argon | Universal standard, especially for refractory elements (Al, Ti, V, B) | Complete inertness; prevents signal suppression | Higher cost than nitrogen |
| Nitrogen | Cost-sensitive routine analysis (for non-refractory elements) | Lower cost | Can form nitrides, suppressing signals for some elements |
Achieve peak performance and sensitivity in your graphite furnace analysis.
Proper gas selection and system control are critical for accurate, reliable results. The experts at KINTEK specialize in lab equipment and consumables, including GFAAS support. We can help you optimize your gas parameters and select the right high-purity gases to protect your investment and ensure data integrity.
Contact us today to discuss your specific application needs and ensure your lab is equipped for success. Reach out to our specialists for a consultation.
Visual Guide
Related Products
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the high temperature graphite material? The Ultimate Solution for Extreme Heat Applications
- What is the role of an industrial graphitization furnace in SiC/MoSi2 coatings? Enhance Substrate Protection
- Why is a graphite furnace more sensitive than a flame? Unlocking Superior Trace Analysis
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment
- What is a graphite furnace? Unlock Extreme Temperatures and Purity for Advanced Materials
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- What is the principle of graphite furnace? Achieve Extreme Temperatures with Direct Resistive Heating
- What is the purpose of carbonization? Transform Organic Materials into Valuable Carbon Products



















