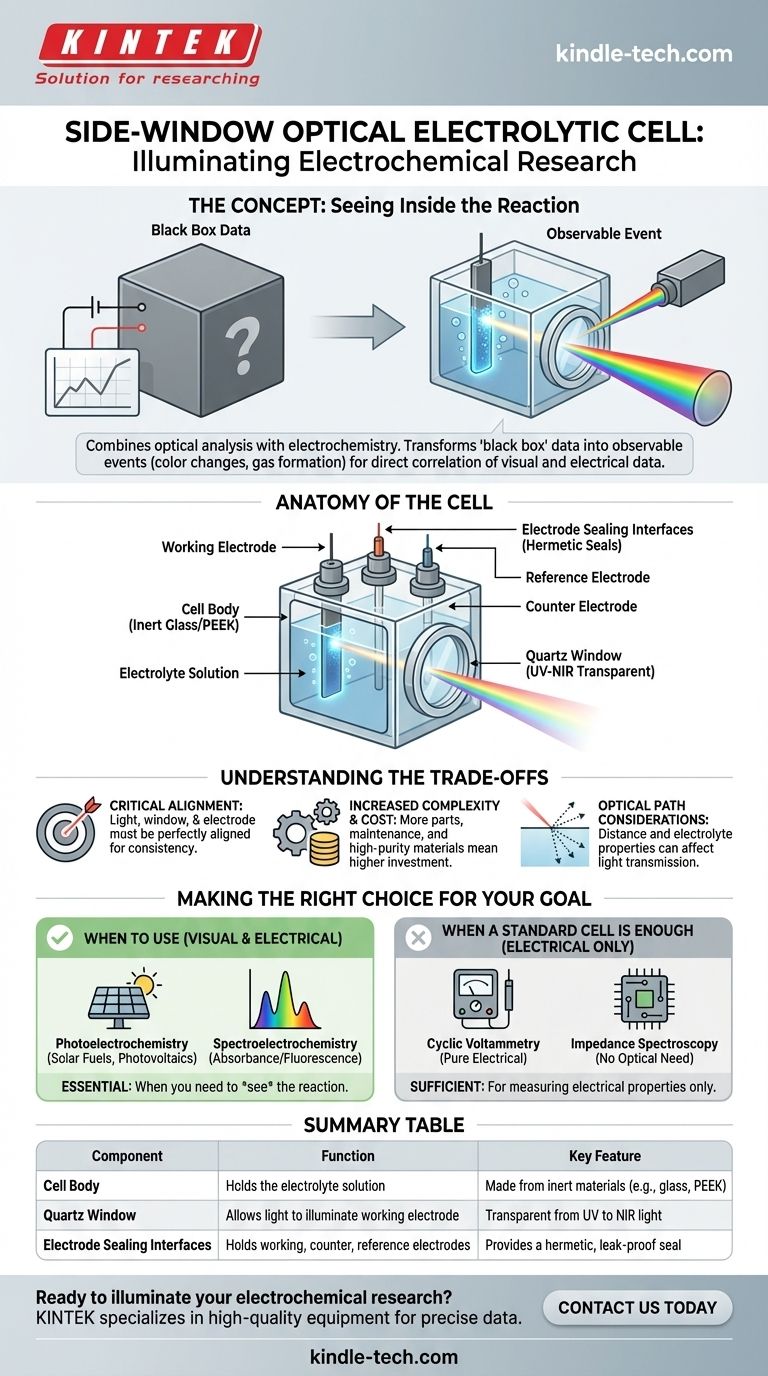
In electrochemical research, a side-window optical electrolytic cell is a specialized vessel designed for experiments that merge optical analysis with electrochemistry. Its defining feature is a transparent quartz window built into its side, which allows a researcher to shine light onto an electrode and simultaneously measure the electrical response and observe optical changes on its surface. This design makes it an indispensable tool for studying light-driven chemical processes.
The true value of a side-window cell is its ability to transform an electrochemical experiment from a "black box" of electrical data into an observable event. It allows you to directly correlate what you see (like color changes or gas formation) with what you measure (current and voltage).

The Problem: Seeing Inside the Reaction
In a standard electrochemical setup, you can precisely control and measure electrical parameters like voltage and current. However, you often cannot see what is physically happening on the electrode's surface where the reaction occurs.
This limitation makes it difficult to answer critical questions about film formation, degradation, color changes (electrochromism), or the efficiency of light-induced reactions. The side-window cell directly solves this by creating a clear line of sight to the action.
Anatomy of the Cell
A side-window cell is an assembly of precision components, each with a critical role in ensuring the integrity of the experiment.
The Cell Body
The body is the main container that holds the electrolyte solution. It is typically made from inert materials like glass or PEEK (polyether ether ketone) to prevent any unwanted chemical reactions with the solution.
The Quartz Window
This is the most critical feature. The window is made of high-purity quartz glass because it is transparent across a broad spectrum of light, from ultraviolet (UV) to visible and near-infrared (NIR). This allows researchers to use various light sources and spectroscopic techniques.
The window’s purpose is to allow a controlled beam of light to illuminate the surface of the working electrode without being obstructed.
Electrode Sealing Interfaces
These are ports designed to hold the three main electrodes of an electrochemical experiment: the working electrode, the counter electrode, and the reference electrode.
These interfaces use gaskets or O-rings to create a hermetic seal. This prevents electrolyte leakage and ensures that each electrode is held in a fixed, reproducible position, which is essential for stable and accurate measurements.
Understanding the Trade-offs
While powerful, these specialized cells introduce complexities that researchers must manage.
Alignment is Critical
The light source, the quartz window, and the surface of the working electrode must be perfectly aligned. Even minor misalignment can cause the light to miss its target, leading to inconsistent or meaningless data.
Increased Complexity and Cost
Compared to a simple glass beaker, a side-window cell has more parts to assemble, clean, and maintain. The high-purity quartz and precision machining also make it a more significant financial investment.
Optical Path Considerations
The distance between the window and the electrode, and the properties of the electrolyte itself, can affect the light beam through scattering or absorption. These factors must be accounted for or corrected during data analysis.
Making the Right Choice for Your Goal
Selecting the right electrochemical cell depends entirely on the question you are trying to answer.
- If your primary focus is studying light-driven reactions: A side-window cell is essential for any photoelectrochemical experiment, such as testing materials for solar fuel generation or photovoltaics.
- If your primary focus is correlating optical and electrical changes: This cell is required for spectroelectrochemistry, where you measure changes in absorbance or fluorescence on an electrode as you sweep its potential.
- If your primary focus is purely measuring electrical properties: A simpler, less expensive standard electrochemical cell is more than sufficient and far easier to use for cyclic voltammetry or impedance spectroscopy without optical components.
Ultimately, choosing a side-window cell means you need to see what is happening, not just measure its electrical effect.
Summary Table:
| Component | Function | Key Feature |
|---|---|---|
| Cell Body | Holds the electrolyte solution | Made from inert materials (e.g., glass, PEEK) |
| Quartz Window | Allows light to illuminate the working electrode | Transparent from UV to NIR light |
| Electrode Sealing Interfaces | Holds working, counter, and reference electrodes | Provides a hermetic, leak-proof seal |
Ready to illuminate your electrochemical research?
A side-window optical electrolytic cell is essential for experiments where you need to see the reaction, not just measure it. KINTEK specializes in providing high-quality lab equipment, including specialized electrochemical cells, to help researchers like you achieve precise, reliable data.
Contact us today to discuss how our solutions can enhance your photoelectrochemical or spectroelectrochemical studies. Let's build the perfect setup for your lab.
Get in touch with our experts now!
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- Which electrode types are compatible with thin-layer spectroelectrochemical cells? Optimize Your Hardware Fit
- What are the key maintenance and handling procedures for a thin-layer spectroelectrochemical cell? Protect Your Sensitive Lab Equipment
- What general precautions should be taken when using a thin-layer spectroelectrochemical cell? Ensure Accurate Results and Equipment Safety
- What is the correct post-experiment procedure for a thin-layer spectroelectrochemical cell? A Step-by-Step Guide for Lab Safety and Accuracy
- What types and sizes of electrodes are typically configured with a thin-layer spectroelectrochemical cell? Standard Setup for Accurate Analysis


















