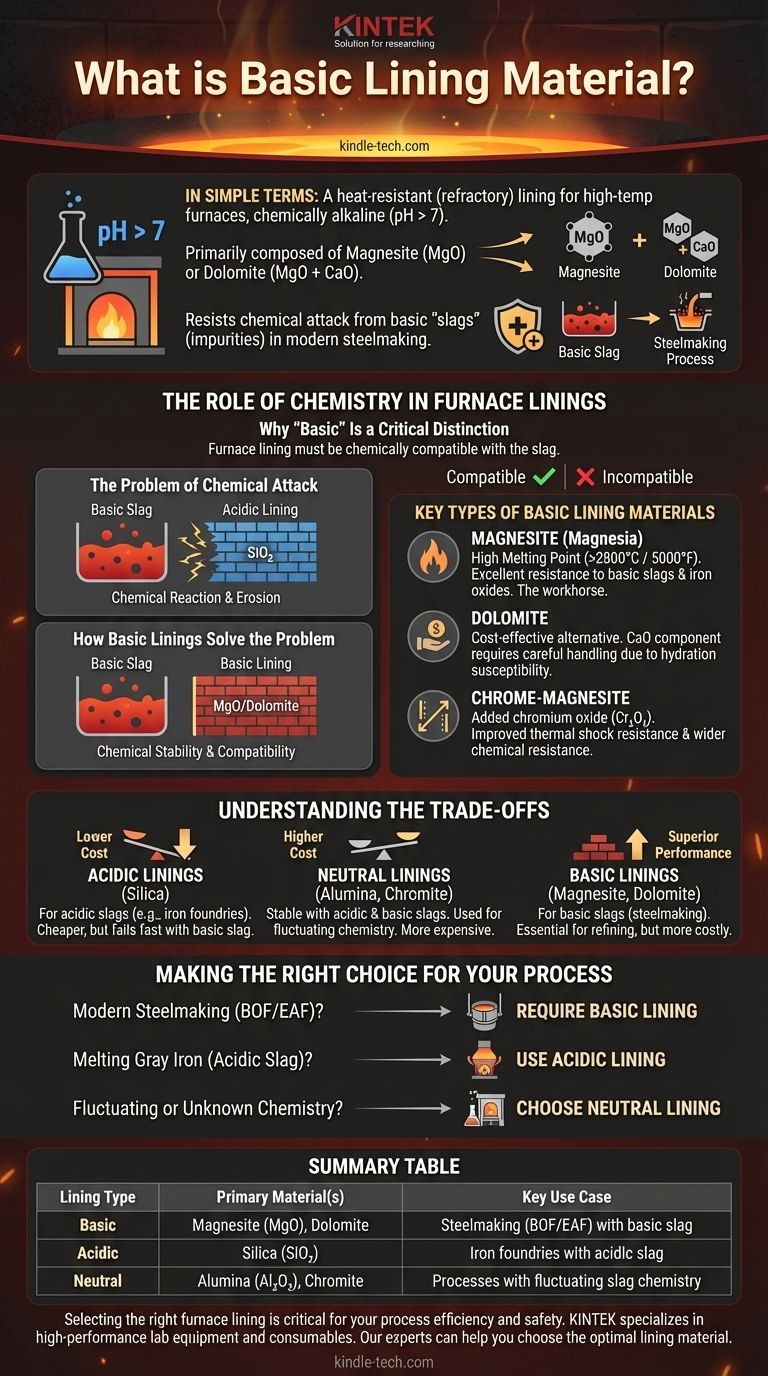
In simple terms, a basic lining material is a type of heat-resistant (refractory) lining used in high-temperature furnaces that is chemically alkaline (with a pH greater than 7). Composed primarily of materials like magnesite (magnesium oxide, MgO) or dolomite (a mix of MgO and calcium oxide), its main purpose is to resist chemical attack from the basic "slags" that are common in modern steelmaking processes.
The term "basic" refers directly to the material's chemical properties, not its simplicity. Selecting the correct lining—acidic, neutral, or basic—is a critical engineering decision based entirely on the chemistry of the high-temperature process it must contain.

The Role of Chemistry in Furnace Linings
Why "Basic" Is a Critical Distinction
In metallurgical processes, molten metal is often covered by a layer of impurities and additives called slag. This slag can be chemically acidic or basic.
The furnace lining must be chemically compatible with the slag it contains. If the lining and the slag have opposite chemical properties (e.g., an acidic lining with basic slag), they will aggressively react with and destroy each other.
The Problem of Chemical Attack
Imagine trying to hold a strong acid in a container made of a reactive metal. The container would quickly corrode and fail. The same principle applies inside a furnace at thousands of degrees.
Using an acidic lining (like silica) with a basic slag would cause a rapid chemical reaction, leading to severe erosion of the furnace wall, compromised safety, and costly production downtime.
How Basic Linings Solve the Problem
Basic lining materials like magnesite are chemically stable and non-reactive when they come into contact with basic slags.
This chemical compatibility is the foundation of modern steelmaking, where basic slags are intentionally used to remove impurities like phosphorus and sulfur from the steel. The basic lining makes this essential refining process possible.
Key Types of Basic Lining Materials
Magnesite (Magnesia)
Based on magnesium oxide (MgO), magnesite is the workhorse of basic refractories. It features a very high melting point (over 2800°C or 5000°F) and excellent resistance to the corrosive effects of basic slags and iron oxides.
Dolomite
Dolomite is a naturally occurring mineral composed of calcium oxide (CaO) and magnesium oxide (MgO). It is typically more cost-effective than high-purity magnesite.
While effective, its calcium oxide component can make it more susceptible to hydration (reacting with moisture from the air), which requires careful handling and storage.
Chrome-Magnesite
For certain applications, chromium oxide (Cr₂O₃) is added to magnesite to improve its properties. This can increase thermal shock resistance and its ability to withstand a wider range of chemical environments.
Understanding the Trade-offs
The Alternative: Acidic Linings
The opposite of a basic lining is an acidic lining, made primarily from silica (SiO₂). These are used for processes that generate acidic slags, such as in some iron foundries using cupola furnaces. They are generally less expensive than basic linings.
The Middle Ground: Neutral Linings
Neutral linings, such as those made from high-purity alumina (Al₂O₃) or chromite, are relatively stable in the presence of both acidic and basic slags. They are often used in applications with fluctuating chemistry or as a buffer zone, but they typically come at a higher cost.
Performance vs. Cost
The choice of lining is always an engineering trade-off. High-purity basic linings offer superior performance in demanding steelmaking applications but are more expensive. An acidic lining is cheaper but would fail instantly in that same environment.
Making the Right Choice for Your Process
The selection of a furnace lining is dictated entirely by the chemistry of your specific operation.
- If your primary focus is modern steelmaking (BOF or EAF): You require a basic lining like magnesite or dolomite to withstand the basic slag used for steel purification.
- If your primary focus is melting gray iron with an acidic slag: An acidic lining made from silica is the standard, cost-effective choice.
- If your primary focus is containing materials with fluctuating or unknown chemistry: A neutral lining like high-alumina brick provides the most versatile chemical resistance.
Understanding this chemical compatibility is the fundamental principle for ensuring efficient, safe, and cost-effective high-temperature operations.
Summary Table:
| Lining Type | Primary Material(s) | Key Use Case |
|---|---|---|
| Basic | Magnesite (MgO), Dolomite | Steelmaking (BOF/EAF) with basic slag |
| Acidic | Silica (SiO₂) | Iron foundries with acidic slag |
| Neutral | Alumina (Al₂O₃), Chromite | Processes with fluctuating slag chemistry |
Selecting the right furnace lining is critical for your process efficiency and safety. KINTEK specializes in high-performance lab equipment and consumables, including refractory solutions for demanding high-temperature applications. Our experts can help you choose the optimal lining material—whether basic, acidic, or neutral—to ensure chemical compatibility, extend equipment life, and maximize your ROI. Contact KINTEK today for a consultation tailored to your laboratory's specific needs!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vertical Laboratory Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What is the sintering time? A Critical Process Variable for Material Density and Strength
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- Why would you braze instead of solder? For Superior Joint Strength and High-Temperature Performance
- How does a high-temperature vacuum sintering furnace facilitate the post-treatment of Zirconia coatings?
- What are the methods of brazing heating? Choose the Right Method for Your Production Needs



















