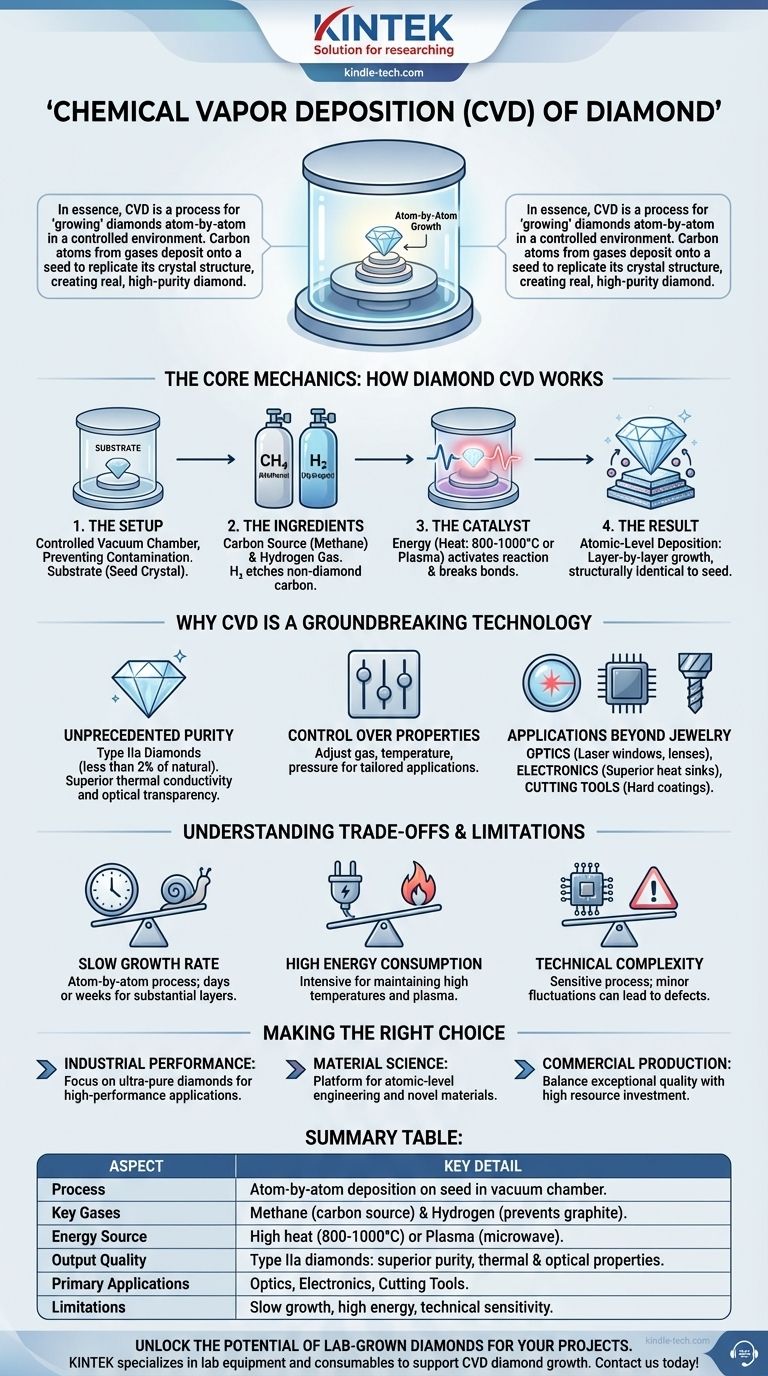
In essence, chemical vapor deposition (CVD) of diamond is a process for 'growing' diamonds atom-by-atom in a controlled environment. By introducing a carbon-rich gas, like methane, into a vacuum chamber containing a substrate (a small 'seed' crystal), high heat or plasma causes the gas to break down. This allows carbon atoms to deposit onto the seed and replicate the diamond’s crystal structure, creating a real, high-purity diamond layer.
CVD is not about creating a mere imitation; it is a sophisticated manufacturing technique that builds a real diamond from the ground up. This process allows for precise control over the material's properties, creating diamonds with exceptional purity and performance for advanced industrial and technological applications.

The Core Mechanics: How Diamond CVD Works
To understand the value of CVD, you must first understand its fundamental process. It is a method of meticulously constructing a solid material from gaseous ingredients, guided by precise environmental conditions.
The Setup: The Chamber and Substrate
The entire process takes place inside a vacuum chamber. This controlled environment is crucial for preventing contamination from unwanted atoms or molecules.
Inside the chamber, a substrate is placed. For growing high-quality single-crystal diamond, this substrate is often a small, pre-existing diamond, sometimes referred to as a "seed crystal."
The Ingredients: Precursor Gases
A carefully controlled mixture of gases is introduced into the chamber. For diamond growth, this typically includes a carbon source gas (most commonly methane, CH₄) and a much larger volume of hydrogen gas (H₂).
The hydrogen plays a critical role: it selectively etches away any carbon that doesn't form a proper diamond bond, ensuring the final product is pure diamond and not graphite.
The Catalyst: Activating the Reaction
The gases alone will not form a diamond. A significant amount of energy is required to break the molecular bonds in the precursor gases, freeing the carbon atoms to deposit on the substrate.
This energy is typically supplied by heating the substrate to very high temperatures (often 800-1000°C) or by using microwaves to generate a plasma—an energized state of gas—within the chamber.
The Result: Atomic-Level Deposition
Once freed, the carbon atoms settle onto the surface of the diamond seed. Under the right conditions, they naturally align with the seed’s existing crystal lattice.
This atomic deposition occurs layer by layer, slowly and precisely building a new diamond that is structurally identical to the seed it grows upon.
Why CVD is a Groundbreaking Technology for Diamond
The ability to grow diamonds is not just about creating gemstones. It's about manufacturing a super-material for applications that natural diamonds cannot fulfill due to impurities, size limitations, or cost.
Unprecedented Purity and Quality
CVD can produce diamonds of exceptional purity, often classified as Type IIa, a category that includes less than 2% of all natural diamonds. This absence of impurities, particularly nitrogen, gives them superior thermal conductivity and optical transparency.
Control Over Material Properties
By carefully adjusting the gas composition, temperature, and pressure during the growth process, technicians can fine-tune the diamond's properties. This allows for the creation of diamonds tailored for specific, demanding applications.
Applications Beyond Jewelry
While used in jewelry, the true impact of CVD diamond is in technology. Its unique properties make it ideal for:
- Optics: Creating durable, highly transparent windows and lenses for lasers and harsh environments.
- Electronics: Acting as a superior heat sink to cool high-power processors and electronics.
- Cutting Tools: Coating industrial tools for exceptional hardness and longevity.
Understanding the Trade-offs and Limitations
Like any advanced manufacturing process, CVD has inherent challenges that are critical to understand. It is a balance of precision, time, and energy.
Slow Growth Rate
Growing diamond atom-by-atom is an inherently slow process. Creating a substantial layer or a single large crystal can take days or even weeks of continuous, stable operation.
High Energy Consumption
Maintaining the extremely high temperatures or generating the plasma required for the chemical reactions is very energy-intensive. This contributes significantly to the operational cost of the process.
Technical Complexity
Diamond CVD is a highly sensitive process. Minor fluctuations in temperature, pressure, or gas purity can lead to defects in the crystal structure or the formation of non-diamond carbon (graphite), compromising the final product's quality.
Making the Right Choice for Your Goal
Understanding CVD for diamond is about recognizing it as a tool for creating an engineered material. Your perspective on its value will depend entirely on your objective.
- If your primary focus is industrial performance: Recognize CVD as the only method to produce ultra-pure diamonds with tailored properties for high-performance optics, thermal management, and cutting applications.
- If your primary focus is material science: View the process as a platform for atomic-level engineering, enabling the creation of novel crystalline materials with properties that do not exist in nature.
- If your primary focus is commercial production: Understand the key trade-off is between the exceptionally high-quality output and the significant energy, time, and capital investment required for the process.
Ultimately, chemical vapor deposition transforms diamond from a rare mineral into a precisely engineered material, opening doors to new technological possibilities.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process | Atom-by-atom deposition of carbon onto a seed crystal in a vacuum chamber. |
| Key Gases | Methane (carbon source) and Hydrogen (prevents graphite formation). |
| Energy Source | High heat (800-1000°C) or plasma (microwave-generated). |
| Output Quality | Type IIa diamonds with superior purity, thermal conductivity, and optical clarity. |
| Primary Applications | Optics (laser windows), electronics (heat sinks), cutting tools. |
| Limitations | Slow growth rate, high energy consumption, technical sensitivity. |
Unlock the potential of lab-grown diamonds for your projects. KINTEK specializes in lab equipment and consumables, providing the tools and expertise to support advanced material synthesis like CVD diamond growth. Whether you're in R&D or industrial manufacturing, our solutions ensure precision and reliability. Contact us today to discuss how we can help you achieve superior material performance!
Visual Guide
Related Products
- Custom CVD Diamond Coating for Lab Applications
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond for Thermal Management Applications
People Also Ask
- What is diamond coating film? A Thin Layer of Diamond for Extreme Performance
- What is Diamond Film? Unlock Extreme Hardness and Thermal Conductivity for Your Applications
- Which is better lab grown or natural diamond? A Clear Guide to Choosing Your Perfect Stone
- What are the uses of diamond in industry? Solving Extreme Engineering Challenges
- Can a jeweler distinguish a lab grown diamond? The Truth About Identifying Diamond Origin
- What is the frequency of MPCVD? A Guide to Choosing 2.45 GHz vs. 915 MHz for Your Application
- How do scientists grow diamonds? Replicating Nature's Process in a Lab
- Are lab diamonds as good as real diamonds? Uncover the Truth About Quality and Value



















