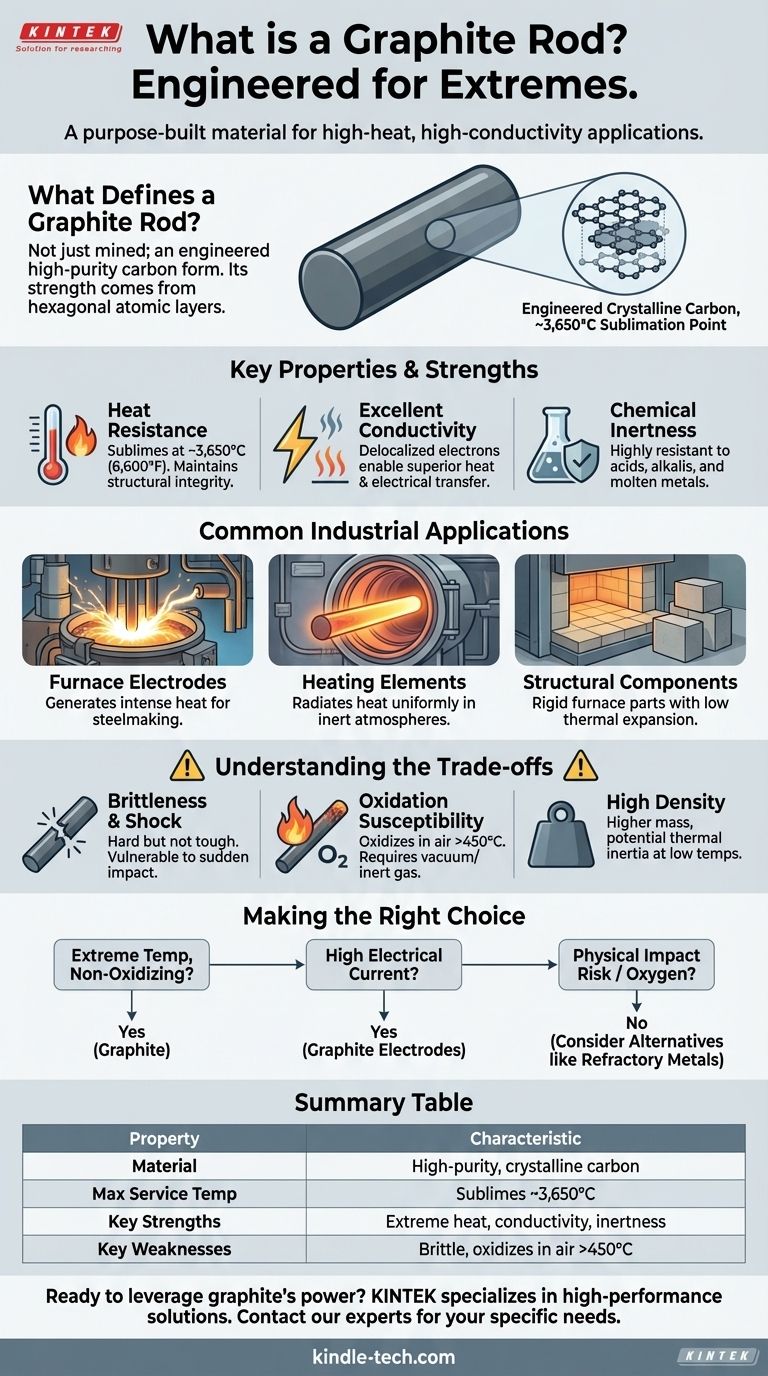
At its core, a graphite rod is a manufactured component made from a high-purity, crystalline form of carbon. It is not simply mined and shaped; it is an engineered material valued for its unique ability to withstand extreme temperatures and conduct heat and electricity, making it indispensable in environments where most metals would melt or degrade.
A graphite rod isn't just a "stick of carbon." It is a purpose-built material designed to perform reliably in extreme industrial environments—specifically high-heat, high-conductivity applications—where conventional materials fail.

What Defines a Graphite Rod?
Graphite's properties are a direct result of its atomic structure. Carbon atoms are arranged in flat, hexagonal layers, similar to a honeycomb. These sheets are strongly bonded internally but weakly bonded to each other.
The Source of Its Strength: Heat Resistance
The primary reason for using graphite is its incredible thermal stability. It does not melt at atmospheric pressure but instead sublimes (turns from a solid directly to a gas) at an exceptionally high temperature, around 3,650°C (6,600°F). This allows it to maintain its structural integrity in furnaces and other high-heat processes.
Excellent Thermal & Electrical Conductivity
The layered structure contains delocalized electrons that move easily, making graphite an excellent conductor of both heat and electricity. This is why it's used for electrodes that carry immense electrical currents and for furnace components, like hearths, that need to distribute heat evenly.
Chemical Inertness
Graphite is highly resistant to chemical attack, especially from acids, alkalis, and molten metals. This makes it an ideal material for containers (crucibles) and fixtures that come into direct contact with corrosive substances at high temperatures.
Common Industrial Applications
The unique combination of these properties makes graphite rods essential in several key industries.
Electrodes for Furnaces
In steelmaking, Electric Arc Furnaces (EAF) use massive graphite electrodes to pass a high-power electric arc through scrap metal, generating the intense heat required to melt it.
Heating Elements
In vacuum or inert-atmosphere furnaces, graphite rods serve as robust heating elements. They can be heated to glowing temperatures to radiate heat uniformly without degrading.
Structural Furnace Components
As noted with graphite hearths, rods and blocks are used to build the internal structures of furnaces. Their rigidity and low thermal expansion mean they maintain their shape and position even under extreme temperature swings.
Understanding the Trade-offs
No material is perfect. To use graphite effectively, you must understand its limitations. Its greatest strengths are also linked to its most significant weaknesses.
Brittleness and Mechanical Shock
Graphite is very hard and rigid, but it is not tough. Unlike a metal that will bend, graphite is brittle and can chip or fracture if subjected to sudden impact or mechanical stress. This requires careful handling and design, especially when loading and unloading furnace parts.
Susceptibility to Oxidation
While it can withstand incredible heat, this is only true in a vacuum or an inert (non-reactive) atmosphere. In the presence of oxygen at high temperatures (typically above 450°C), graphite will oxidize and burn away, essentially turning into CO2 gas and rapidly losing mass.
High Density and Thermal Inertia
Graphite is denser than it appears. This mass means it can sometimes take longer to heat up at lower temperatures compared to a lighter-weight component. However, its excellent thermal conductivity usually helps mitigate this effect by distributing heat quickly once it begins to warm up.
Making the Right Choice for Your Application
Choosing to use graphite is a decision based on balancing its extreme capabilities against its specific vulnerabilities.
- If your primary focus is extreme temperature resistance in a non-oxidizing environment: Graphite is an unparalleled choice for components like heating elements, crucibles, or furnace structures.
- If your application involves high electrical currents for melting or chemical processes: The exceptional electrical conductivity of graphite makes it the industry standard for electrodes.
- If your process involves risk of physical impact or operation in an oxygen-rich atmosphere: You must engineer solutions to protect the graphite or consider alternative materials like refractory metals (e.g., molybdenum, tungsten) if toughness is the most critical factor.
Ultimately, understanding these fundamental properties allows you to leverage graphite's incredible strengths while designing around its weaknesses.
Summary Table:
| Property | Key Characteristic |
|---|---|
| Material | High-purity, crystalline carbon |
| Max Service Temp | Sublimes at ~3,650°C (6,600°F) |
| Key Strengths | Extreme heat resistance, excellent thermal & electrical conductivity, chemical inertness |
| Key Weaknesses | Brittle (susceptible to shock), oxidizes in air above ~450°C |
| Common Uses | Electrodes (EAF), heating elements, furnace structures (hearths), crucibles |
Ready to leverage the power of graphite in your lab or industrial process?
Graphite rods are engineered for reliability in extreme environments where other materials fail. KINTEK specializes in high-performance lab equipment and consumables, including precision graphite components designed for superior thermal management and electrical conductivity.
Our experts can help you select the right graphite solution for your specific application, ensuring optimal performance and durability. Contact our team today to discuss your high-temperature needs and discover how KINTEK's solutions can enhance your efficiency and results.
Visual Guide
Related Products
- Large Vertical Graphite Vacuum Graphitization Furnace
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Platinum Auxiliary Electrode for Laboratory Use
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
People Also Ask
- How does resistance create heat? Unlock the Science of Efficient Energy Conversion
- Why are high-power electric heating rods used in in-situ catalyst reaction cells? Ensure Precision & Thermal Stability
- What are the uses of silicon carbide rod? The Ultimate Heating Solution for Extreme Temperatures
- How do precision resistance heating systems and temperature controllers affect Napier grass carbonization quality?
- What is the temperature range of a MoSi2 heating element? Unlock 1900°C Performance for Your Lab
- What is the best substitute for tungsten? Choose the Right Material for Your Application
- Which material is suitable for use in the heating element? Match the Right Material to Your Application
- What metal is used in heating elements? A Guide to Materials from Nichrome to Tungsten

















