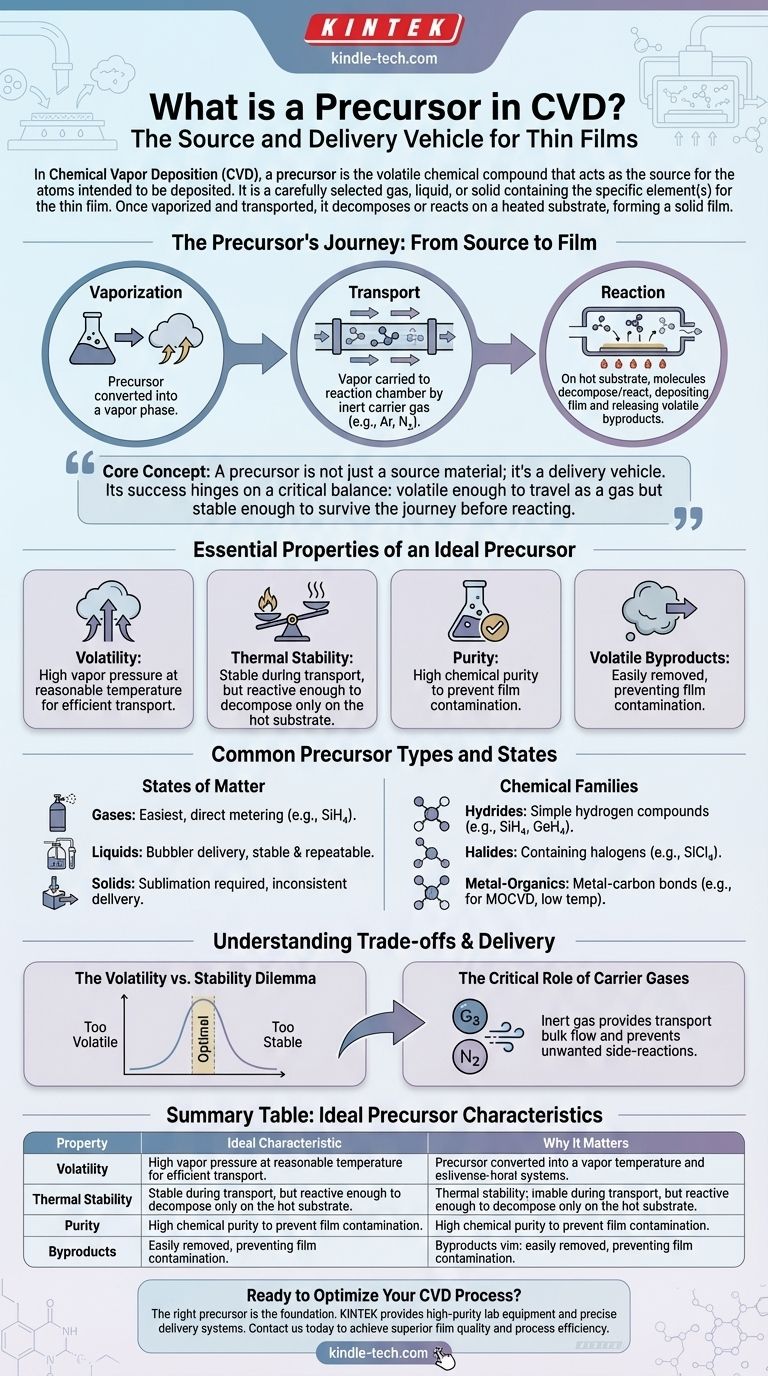
In Chemical Vapor Deposition (CVD), a precursor is the volatile chemical compound that acts as the source for the atoms you intend to deposit. It is a carefully selected substance, available as a gas, liquid, or solid, that contains the specific element(s) needed for the thin film. Once vaporized and transported into a reaction chamber, the precursor decomposes or reacts on a heated substrate, leaving behind the desired material and forming a solid film.
The core concept is that a precursor is not just a source material; it's a delivery vehicle. Its success hinges on a critical balance: it must be volatile enough to travel as a gas but stable enough to survive the journey to the substrate before reacting to form the film.

The Role of the Precursor in the CVD Process
To understand the precursor, you must understand its journey. The entire CVD process is designed around the properties and behavior of this single component.
From Source to Film: A Three-Step Journey
The precursor's function can be broken down into three essential stages:
- Vaporization: The precursor, whether solid, liquid, or gas, must be converted into a vapor phase.
- Transport: This vapor is carried into the reaction chamber, often with the help of an inert carrier gas like argon or nitrogen.
- Reaction: On the hot substrate surface, the precursor molecules gain enough energy to react or decompose, depositing the desired element(s) and releasing other parts of the molecule as volatile byproducts.
The "Chemical" in Chemical Vapor Deposition
The precursor is the literal source of the "chemical" in CVD. The process relies on a chemical change. For example, to deposit silicon (Si), one might use Silane (SiH₄) gas as a precursor. On the hot surface, the SiH₄ molecule breaks apart, the Si atom sticks to the surface, and the hydrogen (H₂) is released as a waste gas.
Essential Properties of an Ideal Precursor
Not just any compound can be a precursor. The selection is a deliberate engineering choice based on a strict set of requirements.
Volatility: The Price of Admission
A precursor must be volatile. This means it must have a high enough vapor pressure at a reasonable temperature to be efficiently transported into the reactor. If a precursor cannot be turned into a gas, it cannot be used in CVD.
Thermal Stability: The Balancing Act
This is the most critical trade-off. A precursor must be stable enough to be vaporized and transported without decomposing prematurely. If it breaks down in the delivery lines, it will never reach the substrate. However, it must also be reactive enough to decompose at the desired deposition temperature on the substrate.
Purity and Byproducts
High chemical purity is essential to avoid incorporating contaminants into the final film. Furthermore, the byproducts of the reaction must also be volatile so they can be easily pumped out of the chamber and do not contaminate the film.
Common Precursor Types and States
Precursors are categorized by both their physical state and their chemical family.
States of Matter: Gas, Liquid, and Solid
- Gases: These are the simplest to use, as they can be directly metered into the chamber from a cylinder. Examples include silane (SiH₄) and ammonia (NH₃).
- Liquids: These are vaporized in a device called a "bubbler," where a carrier gas is bubbled through the liquid to pick up vapor. They often offer more stable and repeatable delivery than solids.
- Solids: These typically require sublimation (heating directly to a gas) at high temperatures and/or low pressures. They can be challenging to use due to inconsistent surface area and heat transfer, making vapor delivery rates harder to control.
Common Chemical Families
- Hydrides: Simple compounds containing hydrogen, such as SiH₄ (silane) and GeH₄ (germane).
- Halides: Compounds containing a halogen like chlorine, such as SiCl₄ (silicon tetrachloride).
- Metal-Organics: A broad class containing a metal-carbon bond, including metal alkyls, alkoxides, and carbonyls. These are the foundation of Metal-Organic CVD (MOCVD) and are prized for enabling deposition at lower temperatures.
Understanding the Trade-offs and Delivery
Choosing and handling a precursor involves navigating several practical challenges.
The Volatility vs. Stability Dilemma
The ideal precursor exists in a narrow window. If it's too volatile, it can be difficult to handle and may evaporate before use. If it's too stable, it requires extremely high temperatures to react, which can damage the substrate or limit the application.
The Critical Role of Carrier Gases
Precursor vapors are rarely used at full concentration. They are diluted in an inert carrier gas (e.g., argon, nitrogen, helium) for two key reasons:
- Transport: The carrier gas provides the bulk flow needed to carry the precursor vapor into the chamber at a controlled rate.
- Protection: The inert gas environment prevents the precursor from undergoing unwanted side-reactions, like oxidation, before it reaches the substrate.
Practicality: Solid vs. Liquid Precursors
For non-gaseous precursors, liquids are often preferred over solids. The consistent surface area and efficient heat transfer in a liquid bubbler allow for much more precise and repeatable control over the vapor flow rate compared to the inconsistent sublimation of a solid source.
Making the Right Choice for Your Process
The precursor defines the process window, the film quality, and the equipment required.
- If your primary focus is process simplicity and high-purity elemental films: Gaseous hydrides or halides are often the most direct choice.
- If your primary focus is low-temperature deposition on sensitive substrates: Metal-organic precursors used in MOCVD are the industry standard.
- If your primary focus is repeatable mass production and stable process control: Liquid precursors delivered via a temperature-controlled bubbler generally offer superior performance over solid sources.
Ultimately, selecting the right precursor is the foundational decision that dictates the quality, properties, and feasibility of your entire CVD process.
Summary Table:
| Property | Ideal Characteristic | Why It Matters |
|---|---|---|
| Volatility | High vapor pressure at reasonable temperature | Ensures efficient transport into the reaction chamber as a gas. |
| Thermal Stability | Stable during transport, reactive at substrate | Prevents premature decomposition; ensures reaction only on the hot surface. |
| Purity | High chemical purity | Avoids contamination of the final thin film. |
| Byproducts | Must be volatile gases | Allows easy removal from the chamber, preventing film contamination. |
Ready to Optimize Your CVD Process?
The right precursor is the foundation of a successful deposition. KINTEK specializes in providing high-purity lab equipment and consumables, including the precise delivery systems needed for gaseous, liquid, and solid precursors. Our experts can help you select the right materials and tools to achieve superior film quality, repeatable results, and process efficiency.
Contact us today to discuss your specific application and let KINTEK be your partner in precision.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained



















