In the context of porcelain, sintering is the critical firing process that transforms a fragile, porous object made of clay particles into a dense, strong, and vitrified final piece. This transformation happens through intense heat, which causes the individual particles to fuse together into a single, solid mass without ever reaching the material's full melting point.
Sintering is not simply about heating the material. It is a controlled, atomic-level process that eliminates the empty spaces between particles, fundamentally changing the porcelain's internal structure to give it its signature strength and non-porous, glass-like quality.

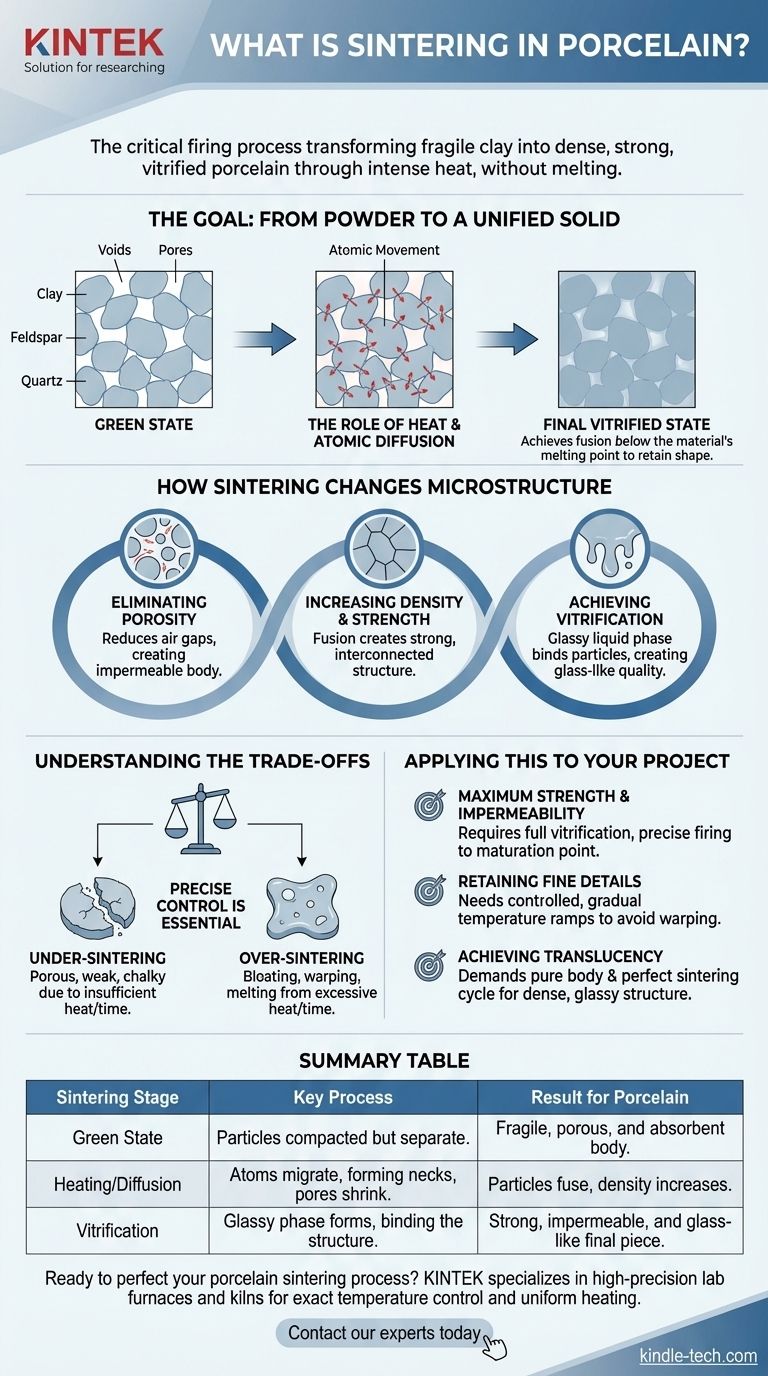
The Goal: From Powder to a Unified Solid
The "Green" State
Before firing, a porcelain object is in its "green" state. It's essentially a compacted mass of individual clay, feldspar, and quartz particles held together by weak mechanical bonds, making it extremely fragile.
The Role of Heat and Atomic Diffusion
During sintering, intense heat energizes the atoms within these particles. This energy causes atoms to migrate, or diffuse, across the boundaries where particles touch.
This atomic movement effectively builds "necks" or bridges between adjacent particles. As the process continues, these necks grow, pulling the particles closer together and systematically eliminating the air-filled pores between them.
Why "Without Melting" Is Critical
The key to sintering is that it achieves this fusion below the material's full melting temperature. If the porcelain were to melt completely, it would lose its shape and collapse into a puddle.
Sintering allows the piece to retain its intended form while undergoing a radical increase in density and strength.
How Sintering Changes Porcelain's Microstructure
Eliminating Porosity
The most significant change during sintering is the reduction and elimination of pores. In the green state, these air gaps make the material weak and absorbent. Sintering closes these voids, creating a dense, impermeable body.
Increasing Density and Strength
As pores are eliminated, the material's density increases dramatically. The fusion of grain boundaries creates a strong, interconnected structure that can withstand significant mechanical stress, a defining characteristic of high-quality porcelain.
Achieving Vitrification
For porcelain, a crucial part of sintering is vitrification. This is the process where some components, like feldspar, melt to form a glassy liquid. This liquid phase flows into the remaining pores and acts as a solvent for other particles, like quartz, binding everything together into an exceptionally strong, glass-like structure upon cooling.
Understanding the Trade-offs
Achieving perfect sintering requires precise control, as mistakes can ruin the final product. Understanding the balance between time and temperature is essential.
The Risk of Under-Sintering
If the porcelain is not heated to a high enough temperature or for a long enough time, the sintering process will be incomplete.
The resulting piece will be porous, weak, and chalky. It will lack the strength, durability, and non-absorbent properties of true porcelain.
The Danger of Over-Sintering
Conversely, excessive heat or time can be just as destructive. This can lead to bloating, where trapped gases expand and create bubbles within the body.
It can also cause the piece to warp, slump, or even melt, destroying its intended form. This is why precise temperature control in the kiln is non-negotiable.
Applying This to Your Project
Your goals for the final piece dictate the precision required in the sintering process.
- If your primary focus is maximum strength and impermeability: You must achieve full vitrification, which requires a precise firing schedule that reaches the material's maturation point without over-firing.
- If your primary focus is retaining fine details and a complex shape: Your firing schedule needs controlled, gradual temperature increases (ramps) to ensure heat is distributed evenly and to minimize the risk of stress or warping.
- If your primary focus is achieving translucency: This demands both a highly pure porcelain body and a perfect sintering cycle that creates a dense, glassy internal structure to allow light to pass through.
Mastering the principles of sintering is the key to unlocking the full potential of porcelain.
Summary Table:
| Sintering Stage | Key Process | Result for Porcelain |
|---|---|---|
| Green State | Particles are compacted but separate. | Fragile, porous, and absorbent body. |
| Heating/Diffusion | Atoms migrate, forming necks between particles. | Particles fuse, pores shrink, density increases. |
| Vitrification | Glassy phase forms from feldspar, binding the structure. | Strong, impermeable, and glass-like final piece. |
Ready to perfect your porcelain sintering process? KINTEK specializes in high-precision lab furnaces and kilns that deliver the exact temperature control and uniform heating required for flawless vitrification. Whether you're aiming for maximum strength, intricate detail retention, or perfect translucency, our equipment is designed to meet the rigorous demands of laboratory and studio environments. Contact our experts today to find the ideal sintering solution for your project!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the disadvantages of quenching? Managing the Risks of Distortion and Cracking
- Why are high-precision furnaces required for alkali glass electrolytes? Optimize Melt-Quenching Stability
- What is the function of a high-temperature sintering furnace in Ti2Nb10O29 synthesis? Achieve Pure Phase Integration
- How does a vacuum drying oven benefit PANI post-treatment? Preserve Conductivity and Structural Integrity
- What are sintered products? Engineered Materials Built from Powder for Superior Performance
- What are the methods of heat treatment of metals? A Guide to Annealing, Quenching, Tempering & More
- What is the step by step process of case hardening? A Guide to Creating Durable, Wear-Resistant Parts
- Can pyrolysis be done in a furnace? Yes, with the right oxygen-free reactor design.



















