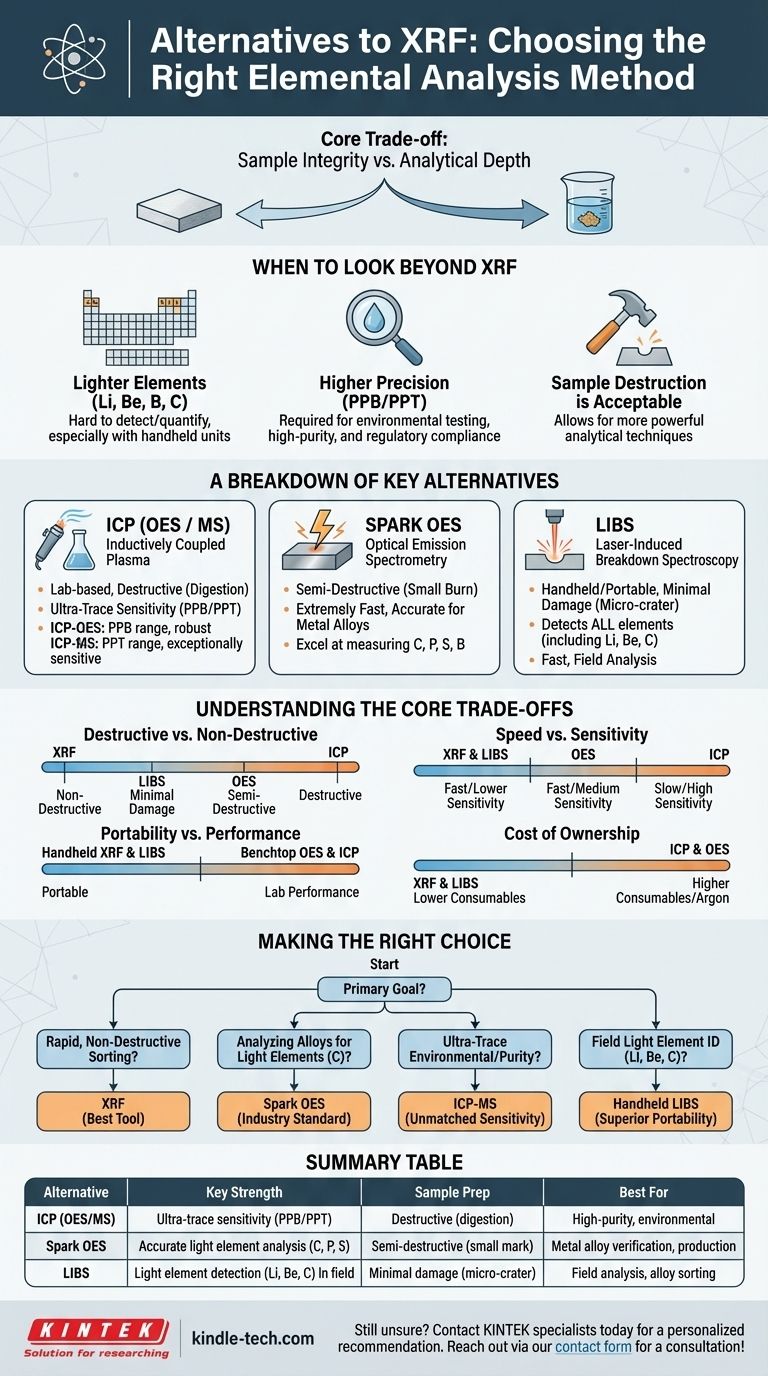
The primary alternatives to X-ray Fluorescence (XRF) are Inductively Coupled Plasma (ICP), Optical Emission Spectrometry (OES), and Laser-Induced Breakdown Spectroscopy (LIBS). Each of these technologies serves a different purpose, and the best alternative depends entirely on your specific need for sensitivity, elemental range, speed, and whether the sample can be destroyed. Choosing the right method is less about finding a direct replacement for XRF and more about matching the technology to the analytical question you need to answer.
The core decision in choosing an alternative to XRF is a trade-off between sample integrity and analytical depth. XRF excels at rapid, non-destructive analysis of solid materials, while its most powerful alternatives require destructive sample preparation to achieve superior sensitivity and a broader elemental range.

When to Look Beyond XRF
XRF is a powerful and versatile tool, but its physical principles create specific limitations. Understanding these limitations is key to knowing when to employ an alternative method.
The Need for Lighter Elements
XRF technology struggles to detect and quantify very light elements. Elements like lithium (Li), beryllium (Be), boron (B), and carbon (C) are either completely undetectable or very difficult to measure accurately with most XRF analyzers, especially handheld units.
If analysis of these specific elements is critical for your application, such as carbon in steel grading or lithium in geological surveys, you must use an alternative.
The Demand for Higher Precision
XRF is excellent for measuring elemental concentrations down to the parts-per-million (PPM) level. However, many applications in environmental testing, high-purity alloy verification, or regulatory compliance require much lower detection limits.
When you need to measure in the parts-per-billion (PPB) range, you have exceeded the practical capabilities of XRF and require a more sensitive lab-based method.
When Sample Destruction is Acceptable
The greatest advantage of XRF is its non-destructive nature. You can analyze a sample and leave it completely intact. However, if your workflow allows for the sample to be destroyed, dissolved, or consumed, a range of more powerful analytical techniques becomes available.
A Breakdown of Key Alternatives
Each alternative technology operates on a different principle, offering a unique set of strengths and weaknesses compared to XRF.
Inductively Coupled Plasma (ICP-OES / ICP-MS)
ICP is a lab-based technique where a sample is first digested in acid and turned into a liquid. This liquid is then nebulized into a fine mist and passed through an extremely hot plasma torch, which excites the atoms.
- ICP-OES (Optical Emission Spectrometry): Analyzes the light emitted by the excited atoms to identify and quantify elements. It is robust and has detection limits in the low PPM to high PPB range.
- ICP-MS (Mass Spectrometry): Separates the ionized atoms by their mass-to-charge ratio. This is an exceptionally sensitive technique, capable of reaching parts-per-billion (PPB) or even parts-per-trillion (PPT) detection limits.
It is the gold standard for trace and ultra-trace elemental analysis but requires a full laboratory, extensive sample preparation, and is the most costly option.
Optical Emission Spectrometry (OES)
Often called "spark OES," this technique is a dominant force in the metals industry. A high-voltage electrical spark is applied to the surface of a metal sample, vaporizing a small amount of material and creating a plasma.
OES is extremely fast and highly accurate for analyzing metal alloys. Crucially, it excels at measuring light elements that are difficult for XRF, such as carbon, phosphorus, sulfur, and boron in steels and other alloys.
Laser-Induced Breakdown Spectroscopy (LIBS)
LIBS operates by firing a high-energy, pulsed laser at the sample surface. The laser ablates a microscopic amount of material, instantly creating a plasma. A spectrometer analyzes the light from this plasma to determine the elemental composition.
Like XRF, LIBS is available in handheld, portable form factors. Its key advantage is the ability to detect all elements, including very light ones like lithium, beryllium, and carbon, that are invisible to most XRF devices.
Understanding the Core Trade-offs
Choosing the right technology requires a clear-eyed assessment of the compromises you are willing to make.
Destructive vs. Non-Destructive
This is the most critical distinction. XRF preserves your sample perfectly. OES creates a small burn mark, and LIBS creates a microscopic crater. ICP, however, requires the complete digestion and destruction of the sample portion being tested.
Speed vs. Sensitivity
Handheld XRF and LIBS provide results in seconds, making them ideal for screening large numbers of samples. In contrast, ICP analysis can take hours or even days when accounting for sample digestion and batch processing, but it delivers unparalleled sensitivity.
Portability vs. Performance
Handheld analyzers (XRF, LIBS) allow for analysis in the field, on a factory floor, or in a warehouse. Benchtop systems (OES, ICP) offer superior performance, stability, and lower detection limits but are confined to a laboratory environment.
Cost of Ownership
The initial purchase price is only one factor. ICP and OES systems require a steady supply of consumable gases (typically pure argon), which adds significant operational cost. XRF and LIBS have much lower consumable costs.
Making the Right Choice for Your Goal
To select the right analytical method, align the technology's strengths with your primary objective.
- If your primary focus is rapid, non-destructive sorting of most alloys: XRF remains the best overall tool for its speed and ease of use.
- If your primary focus is analyzing metal alloys for light elements like carbon: Spark OES is the definitive industry standard for its accuracy and speed in a production environment.
- If your primary focus is ultra-trace environmental or purity analysis: ICP-MS is the only choice for its unmatched parts-per-billion sensitivity, though it requires full sample digestion in a lab.
- If your primary focus is identifying very light elements (Li, Be, C) in the field: Handheld LIBS is the superior technology, providing portability that lab-based methods cannot match.
Understanding these fundamental differences empowers you to choose the analytical tool that delivers the precise data you need, not just the one that is most familiar.
Summary Table:
| Alternative | Key Strength | Sample Prep | Best For |
|---|---|---|---|
| ICP (OES/MS) | Ultra-trace sensitivity (PPB/PPT) | Destructive (digestion) | High-purity materials, environmental testing |
| Spark OES | Accurate light element analysis (C, P, S) | Semi-destructive (small mark) | Metal alloy verification, production control |
| LIBS | Light element detection (Li, Be, C) in field | Minimal damage (micro-crater) | Field analysis, alloy sorting with light elements |
Still unsure which elemental analysis method is right for your lab?
At KINTEK, we specialize in helping laboratories like yours select the perfect equipment for their specific analytical challenges. Whether you need the non-destructive speed of XRF, the light element accuracy of OES, or the trace-level sensitivity of ICP, our experts will guide you to the optimal solution.
We provide high-quality lab equipment and consumables tailored to your workflow, ensuring you get accurate results and maximum return on investment.
Contact our specialists today to discuss your application requirements and get a personalized recommendation. Reach out via our contact form for a consultation!
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Customizable XRD Sample Holders for Diverse Research Applications
- Three-dimensional electromagnetic sieving instrument
- Aluminum Foil Current Collector for Lithium Battery
People Also Ask
- What are the errors in XRF analysis? Master Sample Prep for Reliable Results
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- Why is melting point important for substance identity and purity? A Key Indicator of Sample Composition
- What is the importance of determining the melting point of a substance? Identify Compounds & Assess Purity
- What is the cost of XRF per sample? Find the Right Price for Your Accuracy Needs









