In analytical chemistry, melting point serves as a crucial two-part indicator for any crystalline solid. A pure substance melts at a precise, sharp temperature, a characteristic physical constant. Conversely, the presence of impurities disrupts the substance's crystal structure, causing it to melt at a lower temperature and over a broader range.
The core principle is simple: order requires a specific amount of energy to disrupt. A pure crystal's uniform structure demands a consistent, high energy input to melt, resulting in a sharp, predictable melting point. Impurities introduce disorder, weakening the structure and allowing it to melt with less energy and less uniformity.

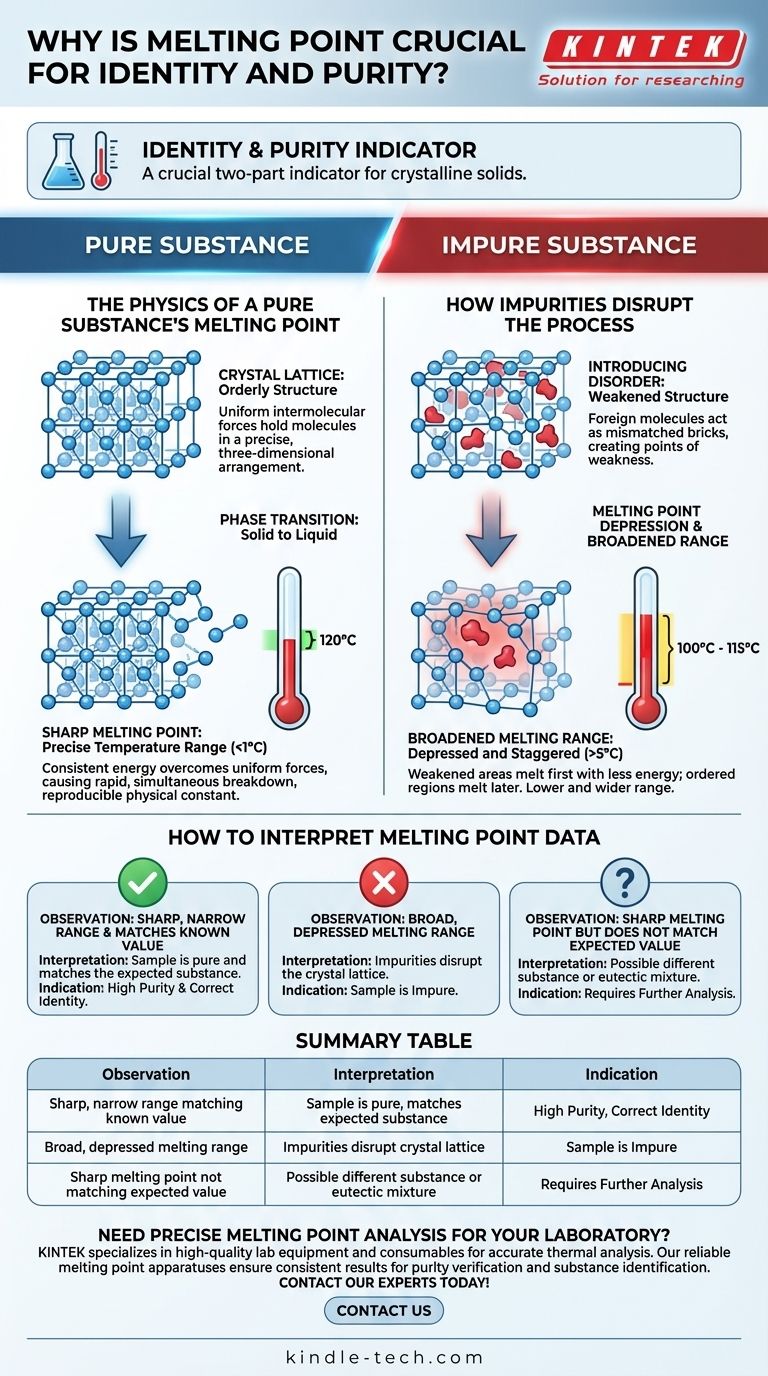
The Physics of a Pure Substance's Melting Point
To understand why melting point is so informative, we must first look at the molecular structure of a pure, crystalline solid.
The Crystal Lattice: An Orderly Structure
Pure crystalline solids are defined by their crystal lattice, a highly ordered, three-dimensional arrangement of molecules. Think of it as a perfectly constructed wall where every brick is identical and placed with precision.
The molecules within this lattice are held in place by intermolecular forces. The strength and uniformity of these forces are consistent throughout the entire crystal.
Energy and Phase Transition
Melting is a phase transition from solid to liquid. This process occurs when enough thermal energy is supplied to the molecules to overcome the intermolecular forces holding them in their fixed lattice positions.
When this happens, the rigid, ordered structure breaks down, and the molecules are free to move past one another in a disordered liquid state.
The "Sharp" Melting Point
Because every part of a pure crystal lattice is essentially the same, the energy required to break it apart is uniform. As you heat the substance, it reaches a specific temperature where the entire structure collapses rapidly.
This results in a "sharp" melting point, which is actually a very narrow temperature range, often less than 1°C. This specific temperature is a reproducible physical constant, like a fingerprint, used to help identify the substance.
How Impurities Disrupt the Process
The presence of even small amounts of an impurity dramatically changes this behavior. This is not a chemical reaction but a physical disruption.
Introducing Disorder
An impurity is a foreign molecule that does not fit into the primary substance's crystal lattice. It acts like a mismatched brick in our wall analogy, creating a point of weakness and disorder.
These defects disrupt the uniform pattern of intermolecular forces, weakening the overall structure in their vicinity.
Melting Point Depression
Because the lattice is now weaker and more disordered, it requires less thermal energy to begin breaking apart. This means the melting process will start at a temperature lower than the melting point of the pure substance.
This phenomenon is known as melting point depression, and it is one of the most reliable indicators of an impure sample.
The Broadened Melting Range
The melting process in an impure sample is not uniform. The areas around the impurities, which are weakest, melt first at a lower temperature.
As you continue to add heat, the more ordered regions of the crystal begin to melt at progressively higher temperatures. The final bit of solid melts at a temperature close to the true melting point of the pure substance.
This staggered process occurs over a wide temperature range (e.g., 5°C or more), resulting in a "broad" melting range. The combination of a depressed and broadened melting range is the classic sign of impurity.
Understanding the Trade-offs and Nuances
While powerful, interpreting melting point data requires an understanding of its limitations and potential exceptions.
The Eutectic Point Exception
It is possible for a specific mixture of two substances to have a sharp melting point, just like a pure compound. This is called a eutectic mixture.
Critically, the melting point of a eutectic mixture is always lower than the melting points of its individual components. If you observe a sharp but unexpectedly low melting point, you might be dealing with a eutectic mixture rather than a pure compound.
Not a Standalone Identifier
Melting point alone is not definitive proof of a substance's identity. Many different compounds can have very similar or identical melting points.
Therefore, melting point is best used as corroborative evidence alongside other analytical techniques like spectroscopy (IR, NMR) or chromatography. A common lab practice is a "mixed melting point" test, where an unknown is mixed with a known sample. If the melting point remains sharp and unchanged, they are likely the same substance. If it becomes depressed and broad, they are different.
The Importance of Technique
The observed melting point can be affected by experimental errors. Heating the sample too quickly can result in a reading that is artificially high and broad because the sample and thermometer do not have time to reach thermal equilibrium. Using too much sample can also broaden the observed range. Accurate, reproducible results depend on careful technique.
How to Interpret Melting Point Data
Your interpretation of the data provides direct insight into the nature of your sample.
- If you observe a sharp, narrow melting range that matches a known value: This is strong evidence that your sample is the expected substance and is highly pure.
- If you observe a broad, depressed melting range: This is a clear indication that your sample is impure. The more the melting point is depressed, the greater the amount of impurity.
- If you observe a sharp melting point that does not match the expected value: Your sample might be a different pure substance entirely, or it could be a specific eutectic mixture, requiring further analysis.
Ultimately, this simple measurement provides a powerful window into the molecular-level composition and order of a material.
Summary Table:
| Observation | Interpretation | Indication |
|---|---|---|
| Sharp, narrow melting range matching known value | Sample is pure and matches expected substance | High purity and correct identity |
| Broad, depressed melting range | Impurities disrupt crystal lattice | Sample is impure |
| Sharp melting point not matching expected value | Possible different substance or eutectic mixture | Requires further analysis |
Need precise melting point analysis for your laboratory? KINTEK specializes in high-quality lab equipment and consumables designed for accurate thermal analysis. Our reliable melting point apparatuses ensure consistent results for purity verification and substance identification. Contact our experts today to find the perfect solution for your laboratory's analytical needs!
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Lab-Scale Vacuum Induction Melting Furnace
- Automatic Laboratory Heat Press Machine
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
- Small Lab Rubber Calendering Machine
People Also Ask
- What are the different types of pyrolysis for biochar? Optimize Your Process for Maximum Yield
- What is the significance of 1°C/min cooling for alloy experiments? Mitigate Stress & Ensure SEM Data Accuracy
- What are the guidelines to follow while heating substances in the laboratory? Ensure Safe and Controlled Heating Processes
- What is the voltage of the e-beam evaporator? Understanding the 4-10 kV Range for Optimal Deposition
- Is sputtering better than evaporation step coverage? Yes, for Superior Coating on Complex Surfaces
- Why is a drying oven used for low-temperature treatment of Ti/Al2O3? Ensure Powder Purity and Flowability
- What is the price range for ultra low temperature freezers? Protect Your Samples with the Right Investment
- What are the uses of sintering? Unlock Manufacturing for High-Temp Materials



















