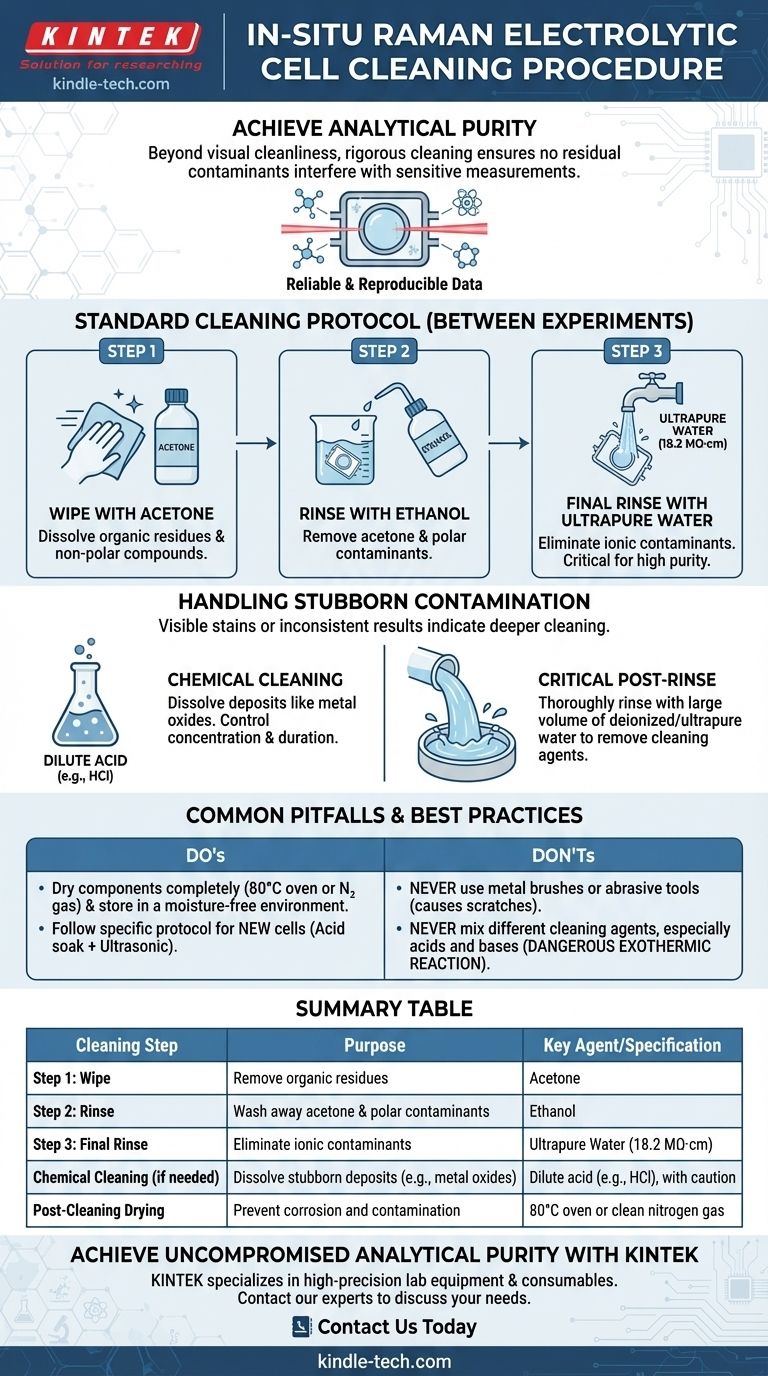
To properly clean a reused in-situ Raman electrolytic cell, the standard procedure is a sequential solvent rinse. First, wipe the inner walls with acetone to dissolve organic residues, then rinse thoroughly with ethanol, and finally complete the process with a rinse using high-purity ultrapure water (18.2 MΩ·cm) to remove any remaining ionic contaminants.
The ultimate goal of cleaning an electrolytic cell extends beyond simple visual cleanliness; it is about achieving analytical purity. A rigorous cleaning protocol ensures that no residual chemicals or contaminants remain to interfere with the sensitive electrochemical and spectroscopic measurements of your next experiment.

The Standard Cleaning Protocol for a Used Cell
This three-step process is the cornerstone of routine maintenance between experiments. It is designed to efficiently remove the most common electrolytes and reaction byproducts without damaging the cell.
Step 1: Wiping with Acetone
Acetone is a powerful organic solvent effective at dissolving a wide range of non-polar compounds, leftover organic electrolytes, and other residues that may not be soluble in water. A gentle wipe of the inner walls is the first critical step.
Step 2: Rinsing with Ethanol
Ethanol is a polar solvent that is miscible with both acetone and water. It serves as an essential intermediate rinse, washing away the acetone and dissolving other polar contaminants that acetone may have missed.
Step 3: Final Rinse with Ultrapure Water
The final, and perhaps most critical, step is rinsing with ultrapure water with a resistivity of 18.2 MΩ·cm. This extremely high purity ensures that no stray ions are deposited onto the cell surfaces, which could otherwise alter the conductivity of your next electrolyte or interfere with your measurements.
Handling Stubborn Contamination
Sometimes, a standard solvent wash is not enough. Visible stains, metal oxide films, or persistently inconsistent experimental results are signs that a more intensive cleaning is required.
Identifying the Need for Deeper Cleaning
If you observe stubborn deposits, such as a rust-colored film from iron oxides, or if your baseline measurements have shifted, it is time for a chemical cleaning procedure. These contaminants can alter electrode surfaces and invalidate your data.
The Chemical Cleaning Process
This process involves using a specific chemical agent to dissolve the deposit. For example, a dilute acid like hydrochloric acid (HCl) can remove metal oxides.
It is crucial to select a chemical that targets the contaminant without corroding the cell material. Always control the concentration and duration of the cleaning to prevent damage.
The Critical Post-Rinse
After any chemical cleaning, you must rinse the cell thoroughly with a large volume of deionized or ultrapure water. This removes every trace of the cleaning agent, which would otherwise become a major contaminant in your next experiment.
Common Pitfalls and Best Practices
Proper handling and awareness of what not to do are just as important as the cleaning procedure itself.
Used Cell vs. New Cell Cleaning
The procedure for a new cell is different and more intensive. It typically involves soaking in a 5% nitric acid (HNO₃) solution for two hours, followed by ultrasonic cleaning in deionized water. This is designed to remove manufacturing oils and residues, not experimental byproducts.
Physical Damage to Avoid
Never use metal brushes or other abrasive tools to scrub the cell. These will create microscopic scratches on the interior surfaces and electrodes. Scratches not only trap future contaminants but can also alter the electrochemical behavior of the system.
Chemical Safety Precautions
It is forbidden to mix different types of cleaning agents, especially acids and bases (like nitric acid and sodium hydroxide). This can trigger a dangerous exothermic reaction, posing a significant safety risk in the lab.
Proper Storage
After cleaning, dry the cell components completely. This can be done in an 80°C oven for one hour or by blowing it dry with clean nitrogen gas. Store the dry cell in a moisture-free environment to prevent corrosion and contamination.
Selecting the Right Cleaning Approach
Your cleaning strategy should match the specific condition of your equipment and your experimental goals.
- If your primary focus is routine, post-experiment cleaning: The acetone, ethanol, and ultrapure water sequence is your standard, reliable procedure.
- If you are facing stubborn deposits or inconsistent results: A targeted chemical cleaning is necessary, followed by an extensive rinse to restore analytical purity.
- If you are preparing a brand new cell for its first use: You must perform the initial acid soak and ultrasonic cleaning to remove all manufacturing residues before its first run.
A meticulously clean cell is the foundation of reliable and reproducible in-situ spectroelectrochemical data.
Summary Table:
| Cleaning Step | Purpose | Key Agent/Specification |
|---|---|---|
| Step 1: Wipe | Remove organic residues | Acetone |
| Step 2: Rinse | Wash away acetone & polar contaminants | Ethanol |
| Step 3: Final Rinse | Eliminate ionic contaminants | Ultrapure Water (18.2 MΩ·cm) |
| Chemical Cleaning (if needed) | Dissolve stubborn deposits (e.g., metal oxides) | Dilute acid (e.g., HCl), with caution |
| Post-Cleaning Drying | Prevent corrosion and contamination | 80°C oven or clean nitrogen gas |
Achieve Uncompromised Analytical Purity with KINTEK
Your in-situ spectroelectrochemical experiments demand a perfectly clean cell for reliable, reproducible data. KINTEK specializes in high-precision lab equipment and consumables, including electrolytic cells designed for easy maintenance and long-term performance.
Let our experts help you select the right equipment and establish best-practice cleaning protocols for your laboratory. Contact us today to discuss your specific needs and ensure your research is built on a foundation of purity and precision.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis



















