The standard cleaning procedure for an electrolytic cell after an aqueous solution experiment is to immediately rinse it thoroughly with deionized water and then dry it with a stream of nitrogen gas. This simple but critical process prevents the deposition of residues that can compromise the integrity of all subsequent experiments.
The core principle is not just cleanliness, but the prevention of cross-contamination. A meticulous cleaning protocol is the foundation for producing accurate, reproducible, and reliable electrochemical data.

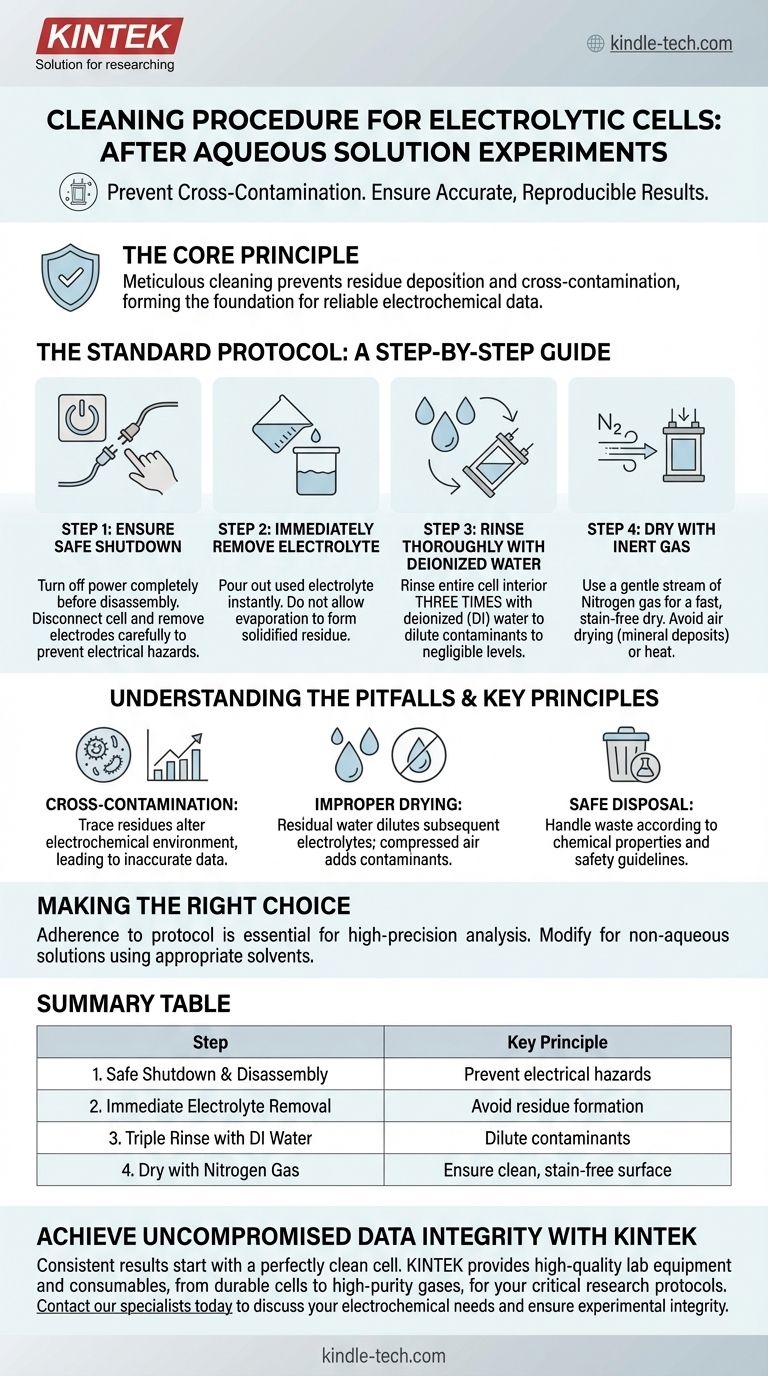
The Standard Protocol: A Step-by-Step Guide
Following a consistent, step-by-step procedure ensures that no contaminants are carried over from one experiment to the next.
Step 1: Ensure Safe Shutdown
Before any disassembly, your first priority is safety. Always turn off the power to the electrochemical workstation completely.
Only after the power is off should you disconnect the electrolytic cell and carefully remove the electrodes. This prevents the risk of electrical arcs or other safety incidents.
Step 2: Immediately Remove the Electrolyte
Once the electrodes are removed, pour the used electrolyte out of the cell without delay.
The most common source of contamination is allowing the electrolyte solution to evaporate, which leaves behind a dried, solidified film of salts and reaction products. Acting immediately prevents this residue from forming.
Step 3: Rinse Thoroughly with Deionized Water
Rinse the entire interior of the cell with deionized (DI) water.
The standard laboratory practice is to perform this rinse three times. This serial rinsing effectively dilutes any remaining contaminants to negligible levels, ensuring the cell is chemically clean.
Step 4: Dry with Inert Gas
After the final rinse, use a gentle stream of nitrogen gas to blow the cell's interior completely dry.
Nitrogen is an inert gas that will not react with the cell surface. This method is superior to air drying, as it is faster and avoids leaving behind water stains, which are essentially mineral deposits that can affect future experiments.
Understanding the Pitfalls and Key Principles
Each step in the cleaning process is designed to mitigate a specific risk to your experimental integrity. Understanding these principles helps reinforce why shortcuts are not advisable.
The Primary Enemy: Cross-Contamination
Even trace amounts of residue from a previous experiment can alter the electrochemical environment of the next one. This can lead to skewed data, inaccurate measurements, and incorrect conclusions. Immediate rinsing is your best defense.
The Problem with Improper Drying
Leaving residual water in the cell can dilute the electrolyte in your next experiment, altering its concentration and conductivity. Using compressed air can introduce oil or particulate contaminants, while heat can damage or stress some cell materials. Nitrogen gas is the clean, safe, and effective standard.
Safe and Responsible Disposal
Your responsibility does not end with cleaning the cell. The waste electrolyte must be handled and disposed of according to its chemical properties and institutional safety guidelines. Never pour reactive or hazardous chemicals down the drain without proper neutralization and approval.
Making the Right Choice for Your Goal
Adherence to this protocol is a mark of a diligent researcher. The level of rigor, however, can be viewed through the lens of your specific objective.
- If your primary focus is high-precision quantitative analysis: This exact procedure is non-negotiable. Any deviation introduces variables that can render your results invalid.
- If your primary focus is qualitative demonstration or teaching: This protocol serves as a critical best practice to instill proper lab discipline and ensure consistent, repeatable outcomes.
- If you are working with non-aqueous or highly reactive solutions: The principles of immediate rinsing and proper drying still apply, but you must use appropriate, non-reactive solvents instead of water.
Ultimately, a clean cell is the silent, essential partner in every successful electrochemical experiment.
Summary Table:
| Step | Action | Key Principle |
|---|---|---|
| 1 | Safe Shutdown & Disassembly | Prevent electrical hazards |
| 2 | Immediate Electrolyte Removal | Avoid residue formation from evaporation |
| 3 | Triple Rinse with Deionized Water | Dilute contaminants to negligible levels |
| 4 | Dry with Nitrogen Gas | Ensure a clean, stain-free surface without contamination |
Achieve Uncompromised Data Integrity with KINTEK
Consistent, reliable results start with a perfectly clean electrolytic cell. At KINTEK, we specialize in providing the high-quality lab equipment and consumables—from durable cells to high-purity gases—that support your critical research protocols.
Let our expertise help you maintain peak laboratory performance. Contact our specialists today to discuss your specific electrochemical needs and ensure the integrity of every experiment.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What core functions does a single-chamber bio-electrochemical reactor provide? Optimize Your MES Experiments Today
- What is the primary function of a Cation Exchange Membrane? Optimize Cu-Cl Cycle Efficiency and Longevity
- What is the primary role of high-precision electrochemical cells in NiTi alloy testing? Ensure Biocompatibility & Safety
- Why is an electrolytic etching system required for Incoloy 800HT? Master Precision Microstructural Visualization
- What type of electrode system is the coating evaluation electrolytic cell designed for? Unlock Precise Coating Analysis
- What are the technical advantages of using a 1-liter three-electrode glass container? Optimize Data Fidelity
- Why is a three-electrode electrochemical cell system necessary for Tafel Extrapolation? Achieve Precision in Corrosion.



















