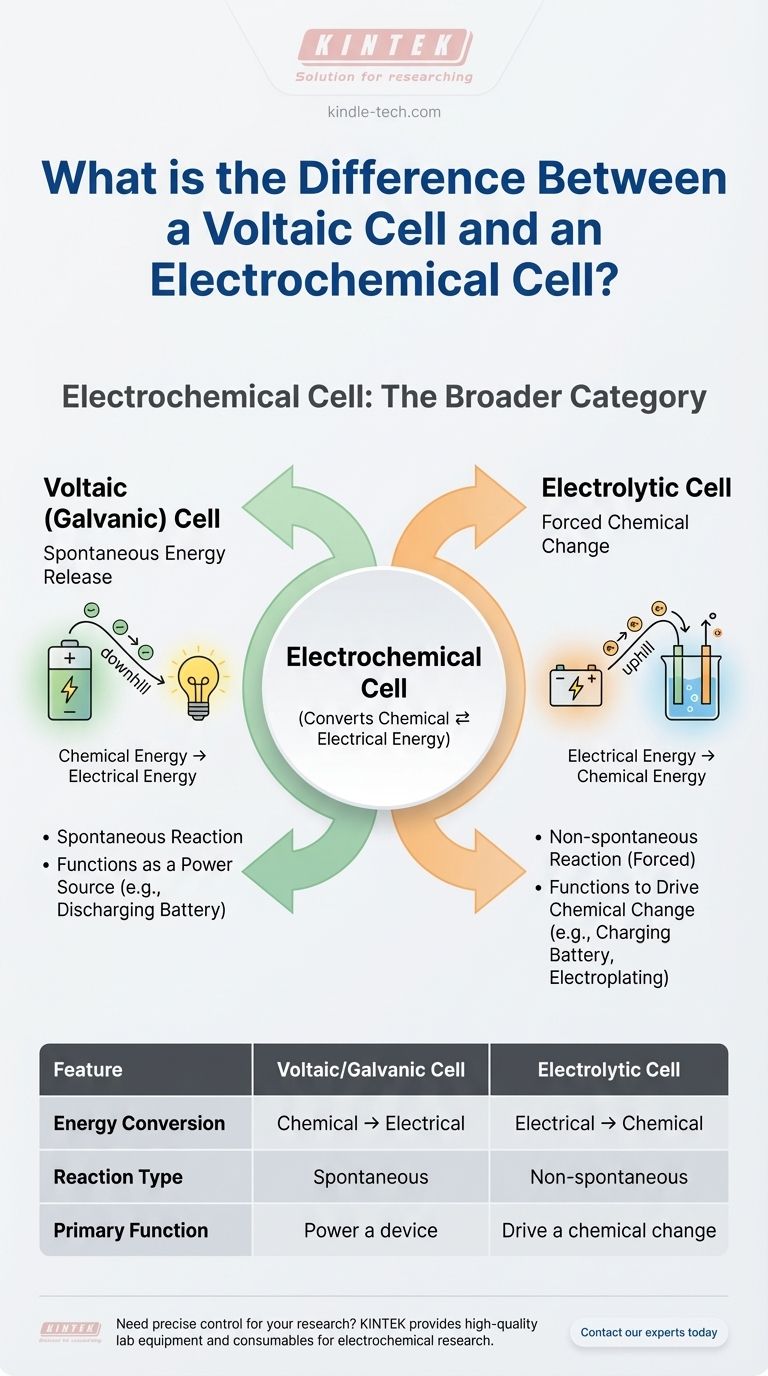
To be direct, a voltaic cell is not different from an electrochemical cell—it is a type of electrochemical cell. The term "electrochemical cell" is the broad category for any device that converts chemical energy into electrical energy or vice versa. A voltaic cell, also known as a galvanic cell, is the specific type that spontaneously generates electricity from a chemical reaction.
The core misunderstanding arises from treating these terms as parallel choices. Instead, think of it as a hierarchy: "Electrochemical Cell" is the family name, and it has two children: the Voltaic Cell (which produces power) and the Electrolytic Cell (which consumes power).
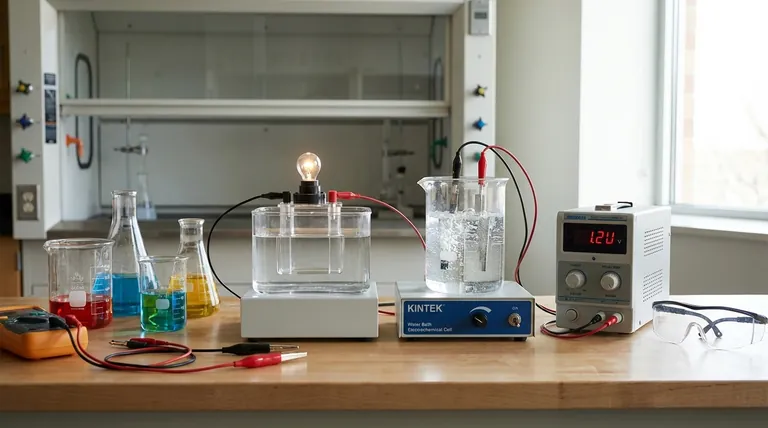
The Two Paths of Electrochemistry
An electrochemical cell is fundamentally a bridge between the chemical and electrical worlds. All such cells share basic components—two electrodes (an anode and a cathode) and an electrolyte that allows ions to move between them.
The crucial difference lies in the direction of the energy conversion.
Type 1: The Voltaic (Galvanic) Cell — Spontaneous Energy Release
A voltaic cell harnesses a spontaneous chemical reaction to produce electrical energy.
Think of it like a controlled slide. The chemicals are naturally "downhill" in terms of energy, and the voltaic cell provides a path for that energy to be released as a useful electric current.
This is the principle behind a common battery. The chemical reactions inside a AA battery want to happen on their own, and when you complete the circuit, the cell channels the resulting flow of electrons to power your device.
Type 2: The Electrolytic Cell — Forced Chemical Change
An electrolytic cell does the exact opposite. It uses an external source of electrical energy to force a chemical reaction that would not happen on its own.
This is the "uphill" path. You are pushing energy into the system to create a less stable chemical state.
Classic examples include using electricity for electrolysis (like splitting water into hydrogen and oxygen) or recharging a battery. When you charge your phone, you are running its battery as an electrolytic cell, reversing the chemical reactions that occurred when it was powering your device.
Understanding the Critical Differences
The distinction between these two cell types is the most important concept in basic electrochemistry. The direction of energy flow dictates the entire function of the cell.
Energy Conversion
A voltaic cell converts chemical energy into electrical energy. It is an energy source.
An electrolytic cell converts electrical energy into chemical energy. It is an energy consumer.
Reaction Spontaneity
The redox reaction in a voltaic cell is spontaneous. It happens without external intervention once the circuit is complete.
The redox reaction in an electrolytic cell is non-spontaneous. It requires an external power supply (like a battery or DC power source) to proceed.
Practical Function
A voltaic cell's function is to power something. Think of any standard, non-rechargeable battery.
An electrolytic cell's function is to produce a chemical change. Think of electroplating metal or charging a rechargeable battery.
How to Classify Your Cell Correctly
To apply this knowledge, simply ask whether the cell is producing power or consuming it to drive a reaction.
- If your primary focus is describing any device that interconverts chemical and electrical energy: Use the broad term electrochemical cell.
- If you are specifically describing a battery that is discharging to power a device: Use the specific terms voltaic cell or galvanic cell.
- If you are specifically describing a process that uses electricity to force a reaction (like charging a battery or electrolysis): Use the specific term electrolytic cell.
By understanding this simple classification, you can describe any electrochemical process with precision and clarity.
Summary Table:
| Feature | Voltaic/Galvanic Cell | Electrolytic Cell |
|---|---|---|
| Energy Conversion | Chemical → Electrical | Electrical → Chemical |
| Reaction Type | Spontaneous | Non-spontaneous (Forced) |
| Primary Function | Power a device (e.g., battery) | Drive a chemical change (e.g., electroplating) |
Need precise control over your electrochemical processes? KINTEK specializes in high-quality lab equipment and consumables for all your electrochemical research and development needs. Whether you're working with voltaic cells, electrolytic cells, or any other laboratory application, our solutions ensure accuracy and reliability. Contact our experts today to find the perfect equipment for your lab!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How should a double-layer water-bath electrolytic cell be operated? A Step-by-Step Guide for Reliable Results
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What is a H type cell? A Guide to Divided Electrochemical Cells for Accurate Experiments
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation



















