At their core, the difference between gold and silver electrodes is a choice between maximum durability and superior cost-efficiency. Gold electrodes are chemically inert, offering exceptional longevity and stable performance, particularly for sensitive, low-frequency measurements. Silver, typically in the form of Silver/Silver Chloride (Ag/AgCl), is the industry standard that provides excellent signal quality for most applications at a significantly lower cost.
The decision is a classic engineering trade-off. Gold is a long-term investment in durability and signal purity for demanding research, while Silver/Silver Chloride offers the best balance of performance and affordability for routine clinical and general-purpose use.

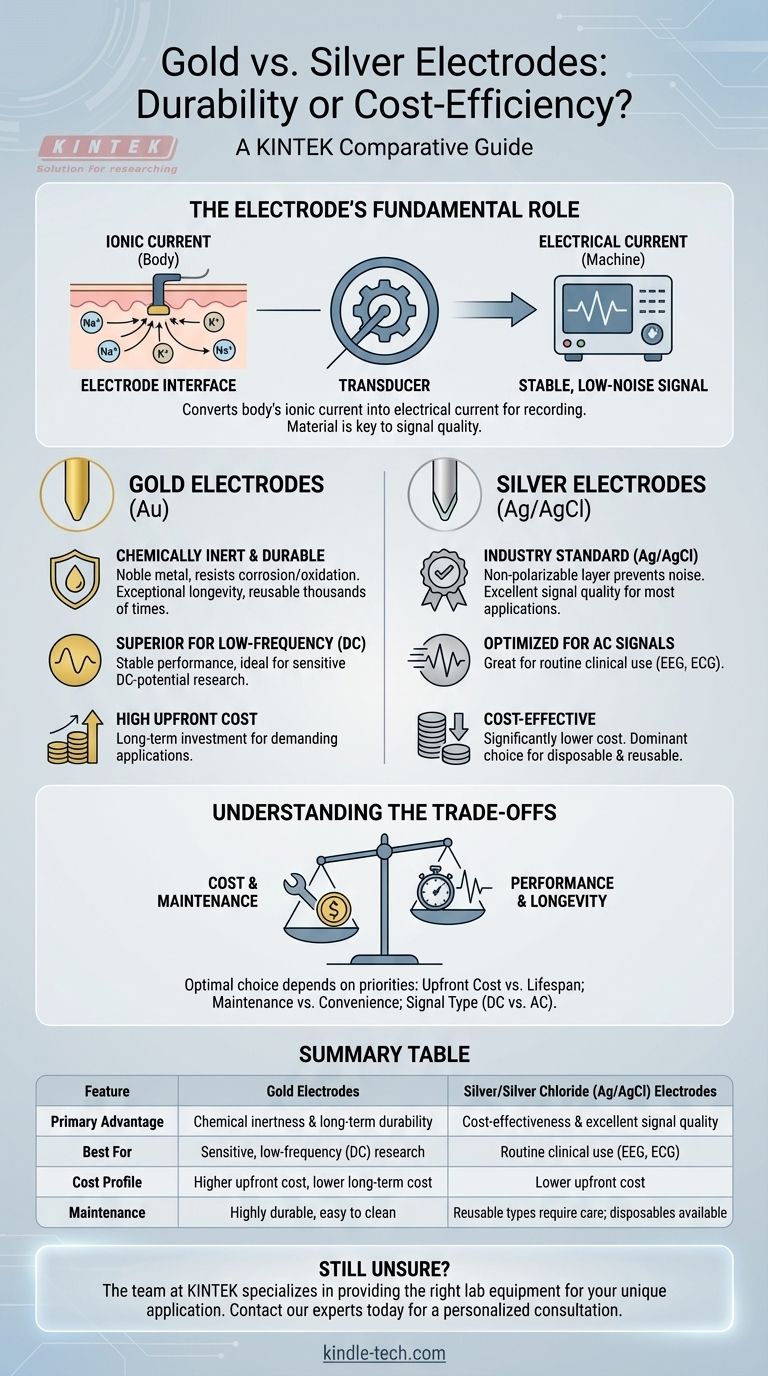
The Electrode's Fundamental Role
To understand the difference, we must first establish the electrode's purpose. In bio-potential measurements like EEG or ECG, the electrode is a transducer.
The Electrode-Skin Interface
An electrode's job is to convert the body's ionic current (the flow of ions like sodium and potassium in tissue) into an electrical current (the flow of electrons in a wire) that a machine can amplify and record.
The Goal: A Stable, Low-Noise Signal
This conversion must happen with as little distortion or noise as possible. The material of the electrode is the single most important factor in determining the quality and stability of this signal conversion.
Analyzing Gold Electrodes
Gold's primary advantage is its chemical nature. It is a noble metal, meaning it is extremely resistant to corrosion and oxidation.
Unmatched Chemical Inertness
Gold electrodes do not react with conductive gels, skin, or cleaning agents. This inertness prevents the buildup of electrochemical noise at the skin interface, which is critical for clean signal acquisition.
Superior Durability and Longevity
Because they do not corrode or degrade, gold electrodes can be used, cleaned, and reused thousands of times without a loss in performance. This makes them highly durable.
Signal Quality for Demanding Applications
The stability of gold makes it the superior choice for measuring very slow-changing biological signals, often called DC potentials. This is a requirement in specific research fields but less critical for standard clinical diagnostics.
Analyzing Silver Electrodes (The Ag/AgCl Standard)
When discussing silver electrodes in a medical context, we are almost always referring to Silver/Silver Chloride (Ag/AgCl), not pure silver.
The Silver/Silver Chloride Advantage
Pure silver electrodes can create their own voltage when interacting with the body, a problem called polarization. By applying a layer of Silver Chloride, the electrode becomes "non-polarizable," allowing current to pass freely without creating noise. This makes Ag/AgCl the gold standard for bio-potential measurement.
Excellent Signal-to-Noise Ratio
For the vast majority of applications, including routine hospital EEGs and ECGs, Ag/AgCl electrodes provide a clean, stable, and reliable signal with an excellent signal-to-noise ratio.
Cost-Effectiveness
Ag/AgCl electrodes are significantly cheaper to manufacture than gold. This has made them the dominant choice for both reusable and single-use disposable electrodes, lowering costs for clinical procedures.
Understanding the Trade-offs
Neither electrode is universally "better." The optimal choice depends entirely on your priorities and application.
Cost vs. Longevity
Gold electrodes have a very high upfront cost but an extremely long lifespan, potentially making them cheaper over many years of heavy use in a research lab. Ag/AgCl electrodes are inexpensive to purchase or replace.
Maintenance and Application
Gold is robust and easy to clean without risk of damage. Reusable Ag/AgCl electrodes can be more delicate, and their chloride layer can degrade over time, while disposable versions eliminate this concern entirely.
Performance in Context
For standard, AC-coupled recordings (most clinical work), the performance difference between a good Ag/AgCl electrode and a gold one is often negligible. The superior stability of gold only becomes a clear advantage in DC-coupled or other sensitive, low-frequency research paradigms.
Making the Right Choice for Your Application
Your final decision should be guided by your specific goal.
- If your primary focus is long-term research or low-frequency recording (DC-EEG): Gold is the superior technical choice due to its unmatched chemical stability and durability.
- If your primary focus is routine clinical use (standard EEG, ECG, or EMG): Silver/Silver Chloride offers the best balance of high-quality signal performance and cost-effectiveness.
- If your primary focus is minimizing upfront investment or using disposables: Silver/Silver Chloride is the clear and logical choice.
Ultimately, understanding the unique demands of your application is the key to selecting the right tool for the job.
Summary Table:
| Feature | Gold Electrodes | Silver/Silver Chloride (Ag/AgCl) Electrodes |
|---|---|---|
| Primary Advantage | Chemical inertness & long-term durability | Cost-effectiveness & excellent signal quality |
| Best For | Sensitive, low-frequency (DC) research | Routine clinical use (EEG, ECG) |
| Cost Profile | Higher upfront cost, lower long-term cost | Lower upfront cost |
| Maintenance | Highly durable, easy to clean | Reusable types require more care; disposable options available |
Still unsure which electrode is right for your specific laboratory needs?
The team at KINTEK specializes in providing the right lab equipment and consumables for your unique application. Whether your priority is the unparalleled durability of gold for long-term research or the cost-effective performance of silver for clinical use, we can help you make the best choice.
Contact our experts today for a personalized consultation and ensure optimal signal quality and value for your lab.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- Why are graphite brushes and carbon felt preferred as anode materials for MECs? Optimize Your Biofuel Performance
- What is the reference electrode value of Ag AgCl? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is commonly used as the anode material? Choosing Between Inert and Active Electrodes
- What are the fundamental characteristics of glassy carbon? Discover its Unique Synergy of Properties
- How should a platinum wire/rod electrode be stored? Protect Your Investment and Ensure Data Accuracy
- What are the recommended maintenance procedures for a glassy carbon sheet? Ensure Reliable Electrochemical Results
- Why is high chemical stability required for carbon foam supports? Ensure Long-Term Durability in Water Electrolysis



















