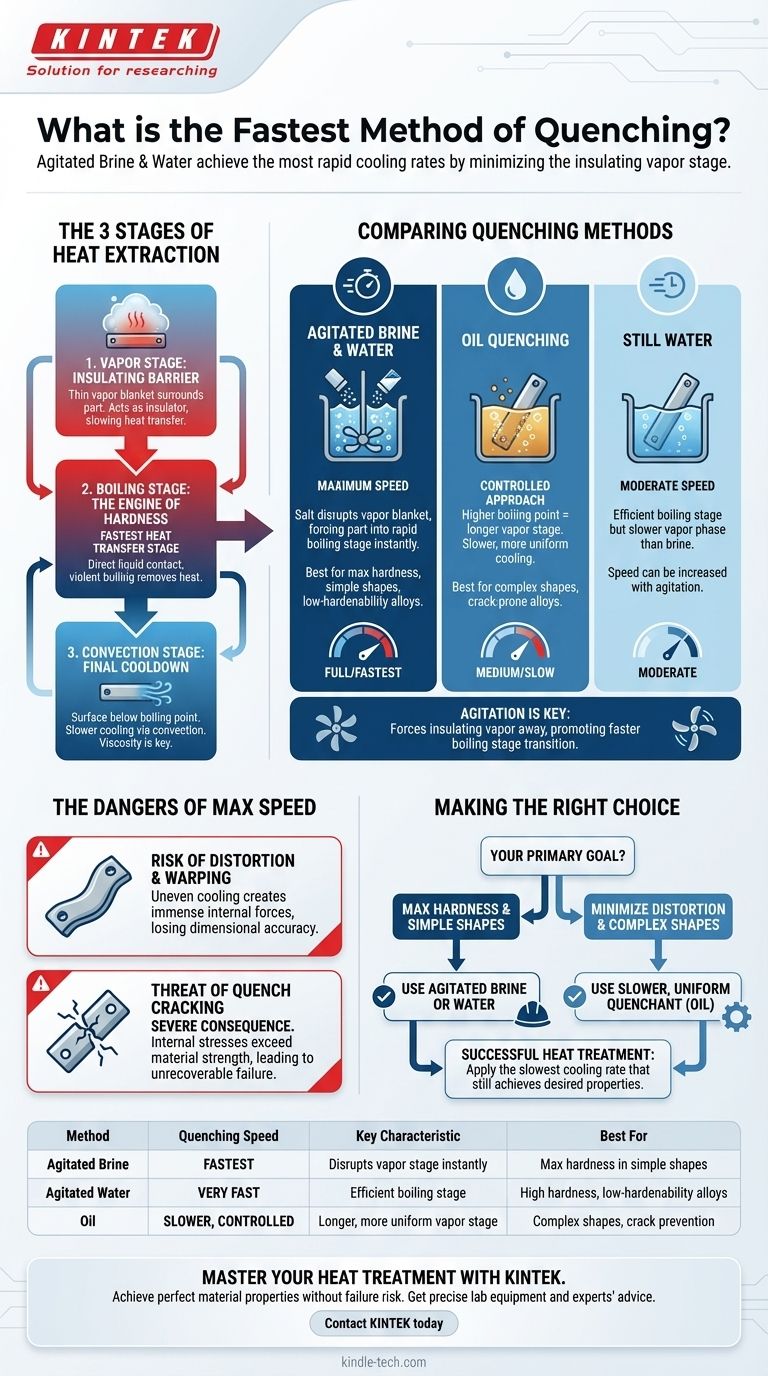
In practice, the fastest method of quenching is typically agitated brine (saltwater), followed closely by agitated water. These methods are designed to extract heat at the most rapid rate possible by aggressively disrupting the initial, slow-cooling vapor stage that insulates the hot component.
The true measure of quenching speed isn't the quenchant itself, but its ability to move a component through the slow, insulating vapor stage and into the extremely rapid boiling stage as quickly as possible. The fastest methods are simply the most effective at achieving this transition.

The Three Stages of Heat Extraction
To understand quenching speed, you must first understand the three distinct phases of heat transfer that occur when a hot part meets a cool liquid. The duration and intensity of each stage dictate the final outcome.
The Vapor Stage: An Insulating Barrier
When the hot component is first submerged, the liquid touching its surface instantly vaporizes.
This creates a thin, stable blanket of vapor that completely surrounds the part. This vapor blanket acts as an insulator, dramatically slowing down heat transfer and cooling.
The Boiling Stage: The Engine of Hardness
As the surface temperature drops slightly, the vapor blanket becomes unstable and collapses.
This initiates the nucleate boiling stage, where the liquid makes direct contact with the component, violently boils, and is thrown away, allowing cooler liquid to rush in. This is by far the fastest stage of heat transfer.
The Convection Stage: The Final Cooldown
Once the component's surface temperature falls below the boiling point of the quenchant, boiling stops.
Cooling continues at a much slower rate through convection, where heat simply moves from the warmer part to the cooler liquid. The viscosity of the quenchant is the primary factor controlling speed in this final stage.
Comparing Common Quenching Methods
Different quenching media and techniques are designed to manipulate these three stages to achieve a desired cooling rate. The "fastest" methods are those that shorten or eliminate the insulating vapor stage.
Water and Brine: Maximum Speed
Water provides a very rapid quench because its boiling stage is extremely efficient at removing heat.
Adding salt to create brine makes the quench even faster. The salt crystals nucleate on the hot surface, disrupting the vapor blanket's formation and forcing the part into the rapid boiling stage almost immediately.
Oil: A More Controlled Approach
Oils have a much higher boiling point than water. This results in a longer, more stable initial vapor stage.
While the overall cooling rate is slower and less severe than water, it is also more uniform. This makes oil a better choice for complex shapes or alloys that are prone to cracking.
The Role of Agitation: Breaking the Barrier
Agitating the quenchant—whether by stirring, pumping, or moving the part—is a critical technique for increasing cooling speed.
Agitation physically forces the insulating vapor blanket away from the component's surface, promoting a faster transition into the highly efficient boiling stage. An agitated oil can be faster than still water in some circumstances.
The Dangers of Maximum Speed
Pursuing the fastest possible quench without understanding the consequences is a common and costly mistake. The most rapid cooling is also the most severe, introducing significant risks.
The Risk of Distortion and Warping
When a component cools at an extreme rate, different sections cool unevenly. The surface cools much faster than the core.
This temperature differential creates immense internal forces that can cause the part to warp, bend, or otherwise lose its required dimensional accuracy.
The Threat of Quench Cracking
Quench cracking is the most severe consequence of excessive cooling speed.
If the internal stresses caused by non-uniform cooling exceed the ultimate strength of the material (especially in its newly hardened, brittle state), the component will crack. This is an unrecoverable failure.
Making the Right Choice for Your Goal
The optimal quenching method is not the fastest one, but the one that achieves the desired metallurgical properties without causing failure. It must be matched to the material's hardenability and the part's geometry.
- If your primary focus is achieving maximum hardness in a simple shape or a low-hardenability alloy: Agitated brine or water is the most effective choice.
- If your primary focus is minimizing distortion and avoiding cracks in a complex shape or a high-hardenability alloy: A slower, less severe quenchant like oil is the safer and more appropriate path.
Ultimately, successful heat treatment depends on applying the slowest cooling rate that will still achieve the necessary material properties for your specific application.
Summary Table:
| Method | Quenching Speed | Key Characteristic | Best For |
|---|---|---|---|
| Agitated Brine | Fastest | Disrupts vapor stage instantly | Maximum hardness in simple shapes |
| Agitated Water | Very Fast | Efficient boiling stage | High hardness, low-hardenability alloys |
| Oil | Slower, Controlled | Longer, more uniform vapor stage | Complex shapes, crack prevention |
Achieve perfect material properties without the risk of failure.
Choosing the right quenching method is critical for the success of your heat treatment process. The wrong choice can lead to cracked, warped components and costly production delays.
KINTEK specializes in providing the precise lab equipment and consumables you need to master your heat treatment processes. Our expertise ensures you can achieve the desired hardness and material integrity for your specific application, whether you're working with simple or complex geometries.
Let our experts help you optimize your quenching process. Contact KINTEK today for a consultation tailored to your laboratory's needs.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- High Performance Laboratory Freeze Dryer
People Also Ask
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- What is an inert condition? A Guide to Preventing Fires and Explosions
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting



















