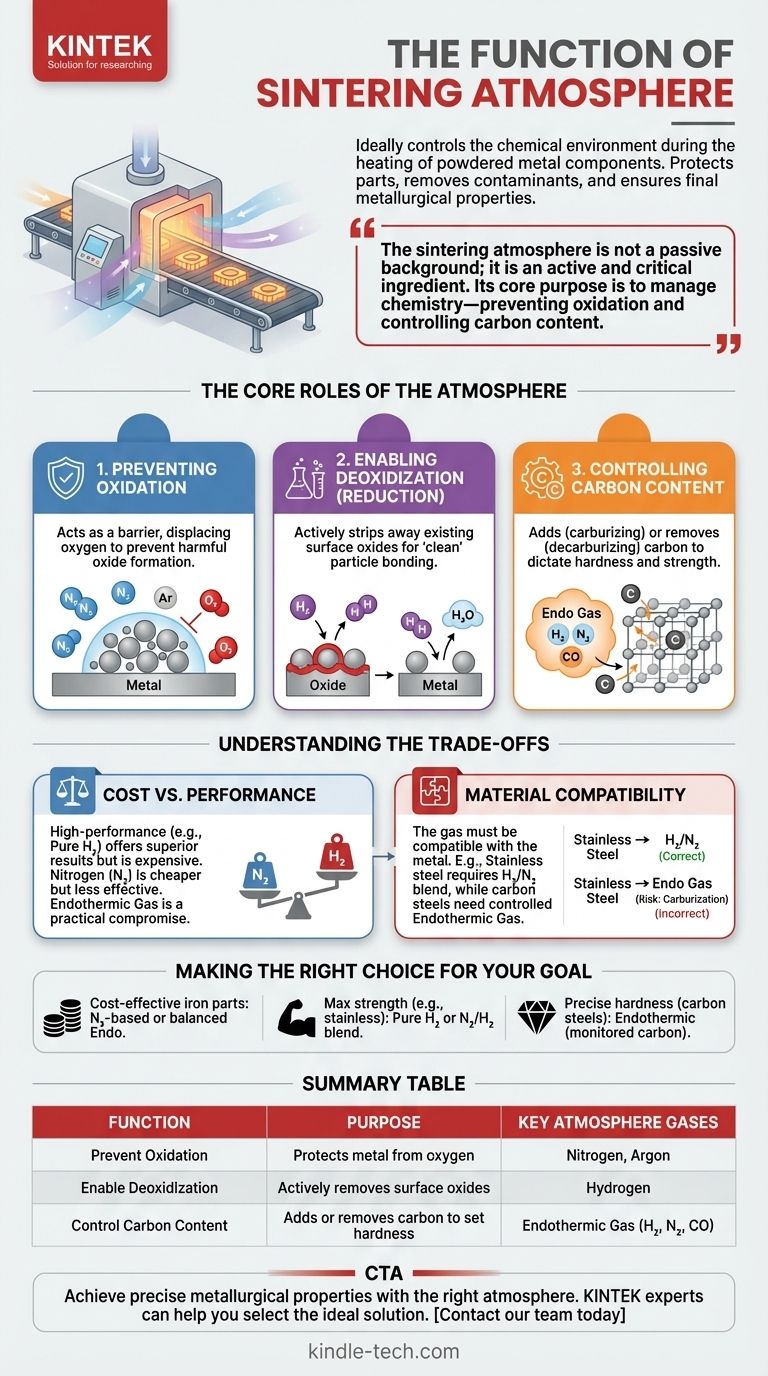
The primary function of a sintering atmosphere is to precisely control the chemical environment during the heating process of powdered metal components. This controlled gas environment protects the parts from harmful reactions, removes existing contaminants like oxides, and ensures the final component achieves its required metallurgical properties.
The sintering atmosphere is not a passive background element; it is an active and critical ingredient in the process. Its core purpose is to manage chemistry—preventing oxidation and controlling carbon content—which directly dictates the final part's strength, integrity, and cost.

The Core Roles of the Sintering Atmosphere
To understand its importance, we must see the atmosphere as a tool that performs several critical jobs simultaneously. Each function is vital for transforming loose powder into a solid, high-integrity component.
Preventing Oxidation
During heating, metal powders are highly susceptible to reacting with oxygen from the air. This forms oxides on the particle surfaces.
These oxide layers act as a barrier, preventing the metal particles from forming strong, direct metallurgical bonds. An inert or reducing atmosphere displaces all oxygen, protecting the parts from this value-destroying reaction.
Enabling Deoxidization (Reduction)
Beyond simple protection, many sintering atmospheres are designed to be chemically reducing. This means they actively strip away oxides that may have already been present on the raw powder.
Gases like hydrogen are powerful reducing agents. They react with and remove surface oxides, effectively cleaning the particles at a microscopic level. This "clean" surface is essential for achieving maximum diffusion and density in the final part.
Controlling Carbon Content
For steel and iron-based components, carbon content is the primary driver of hardness and strength. The sintering atmosphere directly controls this critical variable.
An atmosphere can be carburizing (adding carbon), decarburizing (removing carbon), or neutral. Choosing the wrong atmosphere can accidentally soften a high-strength steel part or make a low-carbon part brittle, completely undermining the material's intended properties.
Understanding the Trade-offs
Selecting a sintering atmosphere is a technical decision balanced by economic reality. The ideal choice depends entirely on the material being processed and the desired outcome, weighed against operational costs.
Cost vs. Performance
High-performance atmospheres deliver superior results but come at a higher price. Pure hydrogen offers the best reducing potential but is expensive and requires significant safety infrastructure.
Nitrogen is a cheaper, inert alternative used for general-purpose protection, but it lacks the oxide-reducing power of hydrogen. Endothermic gas (a mix of hydrogen, nitrogen, and carbon monoxide) often represents a practical compromise, offering good control at a moderate cost.
Material Compatibility
There is no "one-size-fits-all" atmosphere. The chemical composition of the gas must be compatible with the metal being sintered.
For example, using a standard endothermic gas to sinter stainless steel can lead to carburization, compromising its corrosion resistance. In this case, a nitrogen/hydrogen blend or pure hydrogen is required to preserve the material's integrity.
Making the Right Choice for Your Goal
Your choice of atmosphere should be driven by the end-use requirements of the component.
- If your primary focus is cost-effective sintering of non-critical iron parts: A nitrogen-based or balanced endothermic gas provides sufficient protection and control.
- If your primary focus is maximum strength and integrity for sensitive materials like stainless steel: A pure hydrogen or a specific nitrogen/hydrogen blend is necessary to prevent oxidation and unwanted reactions.
- If your primary focus is precise hardness in carbon steels: An endothermic atmosphere with carefully monitored carbon potential is essential to prevent decarburization.
Ultimately, mastering the sintering atmosphere transforms it from a necessary expense into a strategic tool for manufacturing superior components.
Summary Table:
| Function | Purpose | Key Atmosphere Gases |
|---|---|---|
| Prevent Oxidation | Protects metal from reacting with oxygen | Nitrogen, Argon |
| Enable Deoxidization | Actively removes existing surface oxides | Hydrogen |
| Control Carbon Content | Adds or removes carbon to set hardness | Endothermic Gas (H₂, N₂, CO) |
Achieve precise metallurgical properties and cost-effective production with the right sintering atmosphere. KINTEK specializes in lab equipment and consumables for advanced material processing. Our experts can help you select the ideal atmosphere solution for your specific metal powders and performance goals. Contact our team today to optimize your sintering process and manufacture superior components.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- How do atmosphere or vacuum furnaces protect sulfide electrolytes? Key Insights for Safe & High-Performance Synthesis
- What are the primary benefits of using hydrogen firing for sintering parts? Achieve Peak Density & Corrosion Resistance
- Why is an atmosphere furnace required for Carbon-based Solid Acid Catalysts? Mastering Incomplete Carbonization
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2
- What function does a high-temperature atmosphere furnace serve in the activation of Aux/TiO2? Master Catalyst Precision
- What is the use of inert gas in reaction? Control Your Process and Ensure Safety
- What are the different types of sintering atmospheres? Choose the Right One for Your Material
- How are the mixture components calculated for a nitrogen-methanol atmosphere? Essential Ratios for Precise Control



















