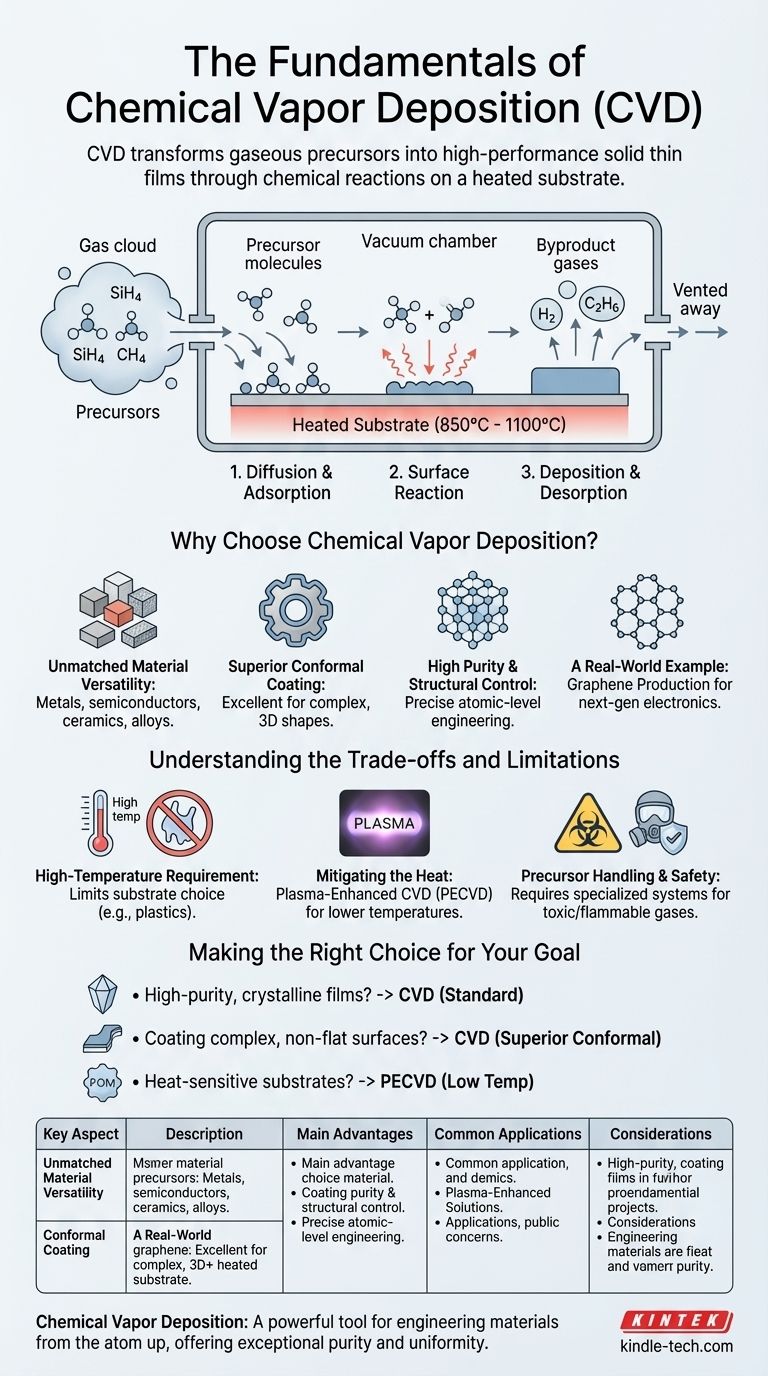
At its core, Chemical Vapor Deposition (CVD) is a process that transforms gases into high-performance solid films. It functions by introducing reactive gas molecules, known as precursors, into a chamber where they decompose or react on a heated surface (the substrate). This chemical reaction results in the formation of a thin, solid material layer on the substrate, with any gaseous byproducts being vented away.
Manufacturing high-quality, uniform thin films is a fundamental challenge in modern technology. Chemical Vapor Deposition provides a solution by offering precise control over a material's growth at the atomic level, enabling the creation of films with exceptional purity and specific structural properties.

How CVD Works: From Gas to Solid Film
The CVD process is a carefully orchestrated sequence of physical and chemical events that occur within a controlled environment. Understanding these steps is key to controlling the final film's characteristics.
The Gaseous Precursors
The building blocks for the film do not start as a solid target. Instead, they are introduced as volatile precursor gases. These precursors contain the specific atoms (e.g., silicon, carbon, titanium) that are intended to form the final solid layer.
The Reaction Chamber
The entire process takes place inside a vacuum chamber where key parameters can be precisely managed. Substrate temperature and chamber pressure are the most critical variables, as they directly influence the speed and nature of the chemical reactions.
The Critical Three-Step Process
While variations exist, the fundamental CVD process unfolds in three main stages on the substrate surface:
- Diffusion and Adsorption: Precursor gas molecules are transported to the substrate and then stick to its surface in a process called adsorption.
- Surface Reaction: Energized by the high temperature of the substrate, the adsorbed molecules undergo a chemical change. This can be a decomposition (breaking apart) or a reaction with other gases to form the desired solid material.
- Deposition and Desorption: The non-volatile, solid product of the reaction deposits onto the substrate, building the film layer by layer. Simultaneously, any volatile byproducts detach from the surface (desorption) and are removed from the chamber.
Why Choose Chemical Vapor Deposition?
CVD is a leading technique for many applications because it offers a combination of versatility and quality that is difficult to achieve with other methods.
Unmatched Material Versatility
CVD is not limited to one type of material. The process can be adapted to deposit a vast range of films, including metals, semiconductors, ceramics, and multi-component alloys.
Superior Conformal Coating
One of CVD's most significant advantages is its excellent "wrap-around" capability. Because the precursor is a gas, it can flow into and coat complex, three-dimensional shapes with a highly uniform film thickness, something line-of-sight methods struggle with.
High Purity and Structural Control
The process yields films with high purity and density. By carefully adjusting parameters like temperature, pressure, and gas flow, an operator can precisely control the film's chemical composition, crystal structure, and grain size.
A Real-World Example: Graphene Production
CVD is a premier method for manufacturing large-area, high-quality graphene. Its ability to produce sheets with a low defect count makes it essential for next-generation electronics, sensors, and high-performance composites.
Understanding the Trade-offs and Limitations
No technique is perfect. Being a trusted advisor means acknowledging the challenges associated with CVD to make an informed decision.
The High-Temperature Requirement
Traditional CVD processes operate at very high temperatures, often between 850°C and 1100°C. This heat is necessary to drive the chemical reactions but means that many substrate materials, such as plastics or certain low-melting-point metals, cannot be used.
Mitigating the Heat
To overcome this limitation, specialized variants have been developed. Plasma-Enhanced CVD (PECVD) uses a plasma to energize the gas precursors, allowing deposition to occur at much lower temperatures and broadening the range of compatible substrates.
Precursor Handling and Safety
The precursor gases used in CVD can be toxic, flammable, or corrosive. This necessitates specialized handling procedures, safety monitoring, and exhaust management systems, which can add complexity and cost to the operation.
Making the Right Choice for Your Goal
Selecting the right deposition technique depends entirely on the requirements of your final product.
- If your primary focus is producing high-purity, crystalline films (e.g., for semiconductors): CVD is an industry-standard choice due to its excellent control over film structure and low defect count.
- If your primary focus is coating complex, non-flat surfaces uniformly: CVD's excellent conformal coverage makes it superior to many line-of-sight deposition methods.
- If your primary focus is working with heat-sensitive substrates (e.g., polymers): Standard high-temperature CVD is unsuitable, and you must investigate lower-temperature variants like Plasma-Enhanced CVD (PECVD).
By understanding these core principles, you can effectively determine when CVD is the ideal tool to engineer materials from the atom up.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Transforms reactive gases into solid films on a heated substrate. |
| Key Steps | 1. Diffusion & Adsorption 2. Surface Reaction 3. Deposition & Desorption |
| Main Advantages | High purity, conformal coating, material versatility, structural control |
| Common Applications | Semiconductor devices, protective coatings, graphene production |
| Considerations | High temperature requirements, precursor safety, equipment complexity |
Ready to engineer materials from the atom up?
Chemical Vapor Deposition is a powerful tool for creating high-performance thin films with exceptional purity and uniformity. Whether you're developing next-generation semiconductors, protective coatings for complex components, or advanced materials like graphene, the right CVD equipment is crucial for success.
At KINTEK, we specialize in providing advanced lab equipment and consumables for all your laboratory needs. Our expertise in CVD technology can help you:
- Achieve precise control over film composition and structure
- Scale your R&D processes to production
- Select the right system configuration for your specific application
Let's discuss how CVD can transform your materials development. Contact our experts today for a personalized consultation!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained



















