The general procedure for operating an in-situ Raman electrolytic cell involves three core phases: meticulous setup, integrated execution, and a safety-conscious shutdown. The process begins with assembling the three-electrode system within the sealed cell, connecting it to an electrochemical workstation, and carefully aligning it with the Raman spectrometer. The experiment is then run by simultaneously applying an electrochemical potential and collecting spectroscopic data, followed by a specific shutdown protocol to ensure safety and data integrity.
Combining electrochemistry with Raman spectroscopy allows for powerful molecular-level insights, but it demands a highly methodical approach. The central principle is to treat the setup not as separate components, but as one integrated system where electrochemical control, spectroscopic alignment, and operational safety are equally critical for success.
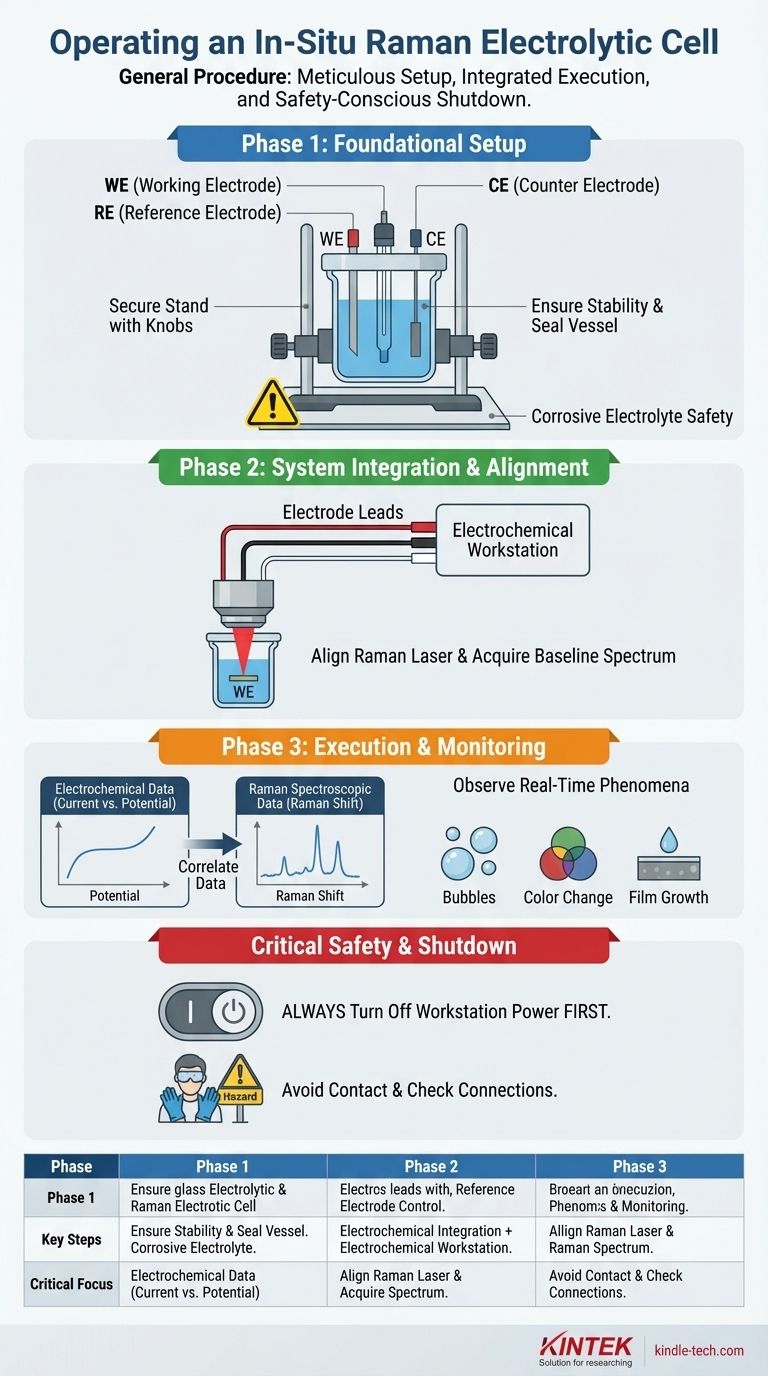
Phase 1: Foundational Setup
The success of your experiment is determined before you ever collect a single data point. This phase is about creating a stable, controlled, and safe environment.
Installing the Three-Electrode System
A standard three-electrode setup consists of a working electrode (WE), a reference electrode (RE), and a counter electrode (CE).
Correctly install these into the reaction vessel. Ensure there is adequate spacing between them to prevent short-circuiting while allowing for uniform current distribution.
Sealing the Reaction Vessel
Once the electrodes are in place, the cell must be tightly sealed. This is crucial for maintaining an inert atmosphere (if needed), preventing electrolyte evaporation, and ensuring a controlled experimental environment.
Ensuring Physical Stability
Place the assembled cell onto its designated stand and tighten any fixing knobs. The cell must be perfectly stable and not wobble, as any movement will disrupt the Raman laser's focus.
If using corrosive electrolytes, place a leak-proof chemical-resistant pad underneath the cell as a critical safety precaution.
Phase 2: System Integration and Alignment
This phase connects the electrochemical and spectroscopic components into a single, functioning analytical system.
Connecting to the Electrochemical Workstation
Connect the electrode leads to the corresponding terminals on your electrochemical workstation. The connections are typically color-coded or labeled: WE, RE, and CE. Double-checking these connections is vital to avoid damaging your equipment or invalidating your results.
Adding the Electrolyte
Carefully add the electrolyte to the cell. The goal is to ensure the active areas of all three electrodes are fully submerged. However, be careful not to overfill; the electrolyte should not touch the external electrode connection points (e.g., alligator clips).
Aligning the Raman Spectrometer
This is the "in-situ" step. Position the electrolytic cell on the Raman microscope stage.
Using the microscope's optics, bring the surface of your working electrode into sharp focus. This is the single most important step for acquiring a strong Raman signal, as the reaction you want to study happens here.
Acquiring a Baseline
Before starting the electrochemical process, acquire a baseline Raman spectrum of the working electrode submerged in the electrolyte. This initial spectrum serves as your "time zero" reference against which all subsequent changes will be measured.
Phase 3: Execution and Monitoring
With the system prepared, you can now run the experiment and collect data.
Setting Parameters and Initiating the Experiment
On the electrochemical workstation software, set your desired parameters, such as the potential scan range, current, or experimental duration.
Once parameters are set, simultaneously start the electrochemical program and the Raman spectral acquisition.
Observing Real-Time Changes
Closely monitor the experiment. Watch for physical phenomena on the electrode surfaces, such as bubble formation, color changes in the electrolyte, or the growth of a film or deposit.
Correlating Observations with Data
The power of this technique comes from correlating your visual observations with the two streams of data you are collecting: the electrochemical data (current vs. potential) and the spectroscopic data (Raman shifts indicating new chemical species).
Critical Safety and Shutdown Procedures
Proper procedure does not end when the data is collected. A disciplined shutdown is essential for safety and equipment longevity.
The Shutdown Sequence
Always turn off the power at the electrochemical workstation first. Only after the potential is off and the system is electronically inactive should you disconnect the electrode leads from the cell. This prevents electrical arcs and potential damage to the workstation.
Handling and Hazards
Throughout the experiment, avoid direct physical contact with the electrodes and electrolyte, which can cause chemical burns or electric shock.
Ensure the experimental area is free of open flames or other ignition sources, especially if your reaction generates flammable gases like hydrogen. Always check that all power cords and connection lines are intact before starting.
Making the Right Choice for Your Goal
Your experimental focus will dictate which steps require the most attention.
- If your primary focus is high-quality spectroscopic data: Dedicate the most time to precisely focusing the laser on the working electrode and ensuring the cell is perfectly stable.
- If your primary focus is accurate electrochemical measurements: Prioritize the correct installation of the three electrodes, ensuring no leaks, and using a stable reference electrode.
- If your primary focus is safety and reproducibility: Master the methodical setup and shutdown sequence, and document every parameter and observation with extreme care.
By mastering this integrated procedure, you transform a complex setup into a powerful tool for discovery.
Summary Table:
| Phase | Key Steps | Critical Focus |
|---|---|---|
| 1. Foundational Setup | Install three-electrode system, seal vessel, ensure stability | Prevent short-circuiting, maintain inert atmosphere |
| 2. System Integration | Connect to electrochemical workstation, align spectrometer, acquire baseline | Precise laser focus on working electrode surface |
| 3. Execution & Shutdown | Run experiment, monitor real-time changes, follow safety shutdown | Correlate electrochemical data with Raman shifts, power off workstation first |
Ready to achieve precise molecular-level insights with your in-situ experiments?
At KINTEK, we specialize in providing high-quality lab equipment and consumables for electrochemical and spectroscopic applications. Whether you're setting up a new in-situ Raman electrolytic cell or optimizing your existing workflow, our experts can help you select the right equipment and ensure optimal performance.
We provide:
- Reliable electrochemical cells and components
- Precision alignment tools for spectroscopic integration
- Safety equipment and consumables for controlled environments
Let us help you enhance your lab's capabilities and data accuracy. Contact our technical specialists today to discuss your specific experimental needs and discover how KINTEK solutions can drive your research forward.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- How does the design of an electrolytic cell influence evaluation of electrochemical catalytic performance? Key Factors
- How does an electrochemical workstation evaluate corrosion resistance of welded joints? Expert Testing Guide
- What personal and environmental safety measures should be taken when operating an electrolysis cell? A Complete Guide to Safe Operation
- What is the applicable temperature range for the H-type electrolytic cell? Mastering Precise Thermal Control
- What are the specifications of the openings on the H-type electrolytic cell? Optimized Port Layout Guide
- How does a high-purity argon protection system contribute to the molten salt electrochemical synthesis process? Ensure Material Phase Purity
- What are the different types of cells in electrolysis? Understanding Electrolytic vs. Galvanic Cells
- What materials are used for an optical electrolytic cell body? Choose the Right Material for Your Experiment



















