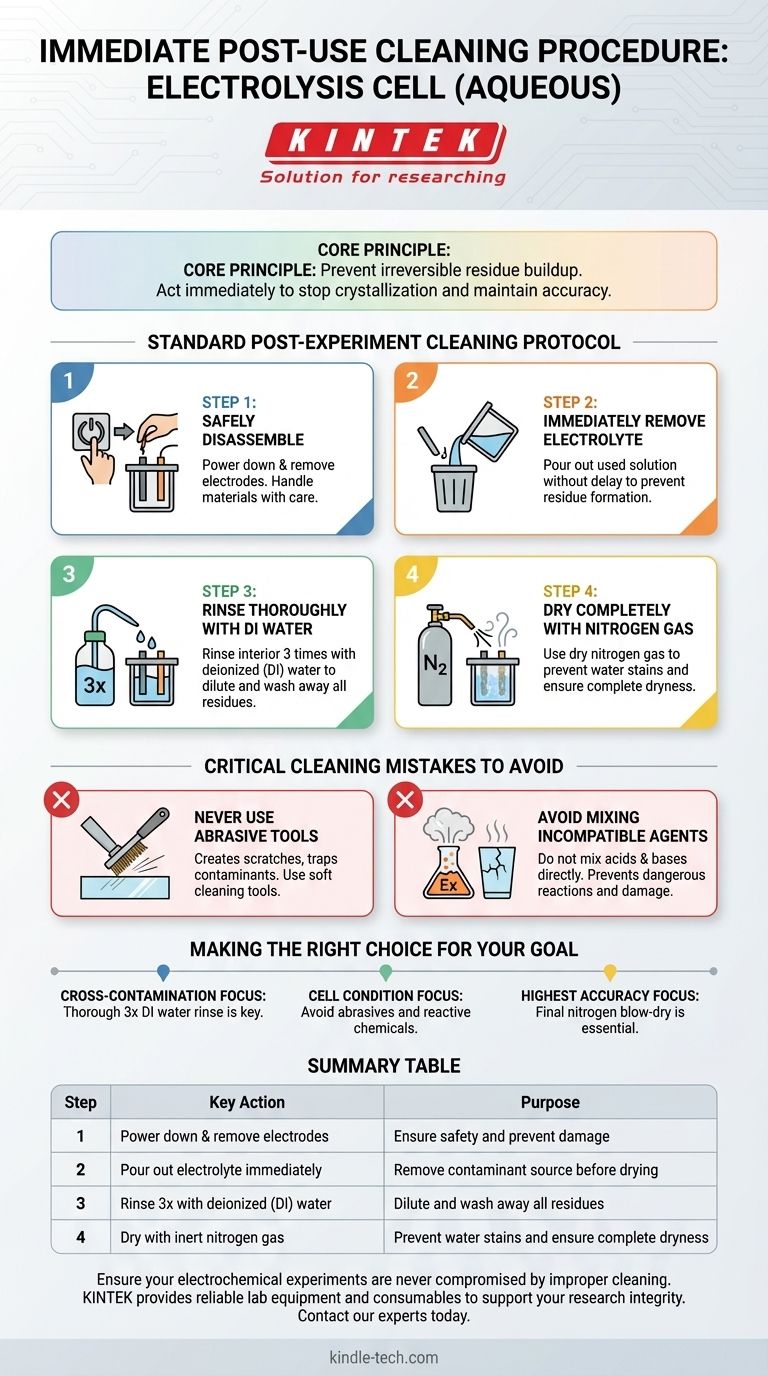
The immediate cleaning procedure for an electrolysis cell involves powering down the equipment, removing the electrodes, pouring out the electrolyte, rinsing the cell thoroughly with deionized water, and finally drying it with nitrogen gas. This sequence is critical to perform immediately after use to prevent residues from drying and contaminating future experiments.
The core principle is not just cleanliness, but the prevention of irreversible residue buildup. Acting immediately after an experiment stops dissolved salts and reaction byproducts from crystallizing or adhering to the cell surfaces, which is essential for maintaining experimental accuracy and equipment longevity.

The Standard Post-Experiment Cleaning Protocol
A disciplined cleaning process is fundamental to reliable electrochemical work. Each step is designed to systematically remove contaminants without damaging the sensitive apparatus.
Step 1: Safely Disassemble the Cell
First, ensure the power supply to the cell is turned off completely. This eliminates any risk of electrical shock or unintended electrochemical reactions during disassembly.
Once powered down, carefully remove the electrodes from the cell. Handle them as specified by their material type, as they may be fragile or require their own specific cleaning protocol.
Step 2: Immediately Remove the Electrolyte
This step is time-sensitive. Pour out the used electrolyte solution from the cell without delay.
The goal is to remove the source of potential contaminants before the solvent (water) evaporates, which can leave behind a difficult-to-remove film of crystalline salts or other residues.
Step 3: Rinse Thoroughly with Deionized Water
Rinse the interior of the cell at least three times with deionized (DI) water. Using DI water is crucial because it is free of the ions found in tap water, which could otherwise deposit onto the cell walls and interfere with subsequent experiments.
The repeated rinsing ensures that any lingering electrolyte is diluted and washed away effectively.
Step 4: Dry Completely with Nitrogen Gas
After the final rinse, use a gentle stream of dry nitrogen gas to blow-dry the interior of the cell.
Nitrogen is an inert gas that will not react with the cell surfaces. This method is superior to air drying as it is faster and prevents the formation of water stains, which are essentially mineral deposits left behind as water evaporates.
Critical Cleaning Mistakes to Avoid
Proper cleaning is as much about what you don't do as what you do. Certain common mistakes can permanently damage the electrolysis cell or compromise safety.
Never Use Abrasive Tools
Do not use metal brushes or other abrasive implements to clean the interior of the cell.
These tools can create microscopic scratches on the glass surface. These scratches are not merely cosmetic; they increase the surface area and create sites where contaminants can become trapped, leading to cross-contamination between experiments.
Avoid Mixing Incompatible Cleaning Agents
Never mix acids and bases, such as nitric acid (HNO₃) and sodium hydroxide (NaOH), directly within the cell for cleaning purposes.
This combination can trigger a highly exothermic reaction, generating significant heat that could crack the glass cell or cause a dangerous pressure buildup. Always follow established protocols for using specific cleaning solutions, and never mix chemicals without understanding their reactivity.
Making the Right Choice for Your Goal
Your cleaning diligence directly impacts the quality of your results. Tailor your focus to your primary experimental objective.
- If your primary focus is preventing cross-contamination: The immediate and thorough three-part rinse with deionized water is the single most important step.
- If your primary focus is preserving the cell's physical condition: Strictly avoid using abrasive tools and mixing reactive cleaning chemicals inside the cell.
- If your primary focus is achieving the highest analytical accuracy: The final nitrogen blow-dry is essential to prevent water spots and residual moisture that could alter concentrations in your next experiment.
A consistent and correct cleaning protocol is the foundation of reliable and repeatable electrochemical research.
Summary Table:
| Step | Key Action | Purpose |
|---|---|---|
| 1 | Power down & remove electrodes | Ensure safety and prevent damage |
| 2 | Pour out electrolyte immediately | Remove contaminant source before drying |
| 3 | Rinse 3x with deionized (DI) water | Dilute and wash away all residues |
| 4 | Dry with inert nitrogen gas | Prevent water stains and ensure complete dryness |
Ensure your electrochemical experiments are never compromised by improper cleaning. KINTEK specializes in providing reliable lab equipment and consumables, including electrolysis cells and high-purity DI water systems, to support your research integrity. Contact our experts today to discuss your specific laboratory needs and how we can help you maintain peak experimental accuracy.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- What are the standard opening specifications for sealed and unsealed all-quartz electrolytic cells? Optimize Your Electrochemistry Setup
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab



















