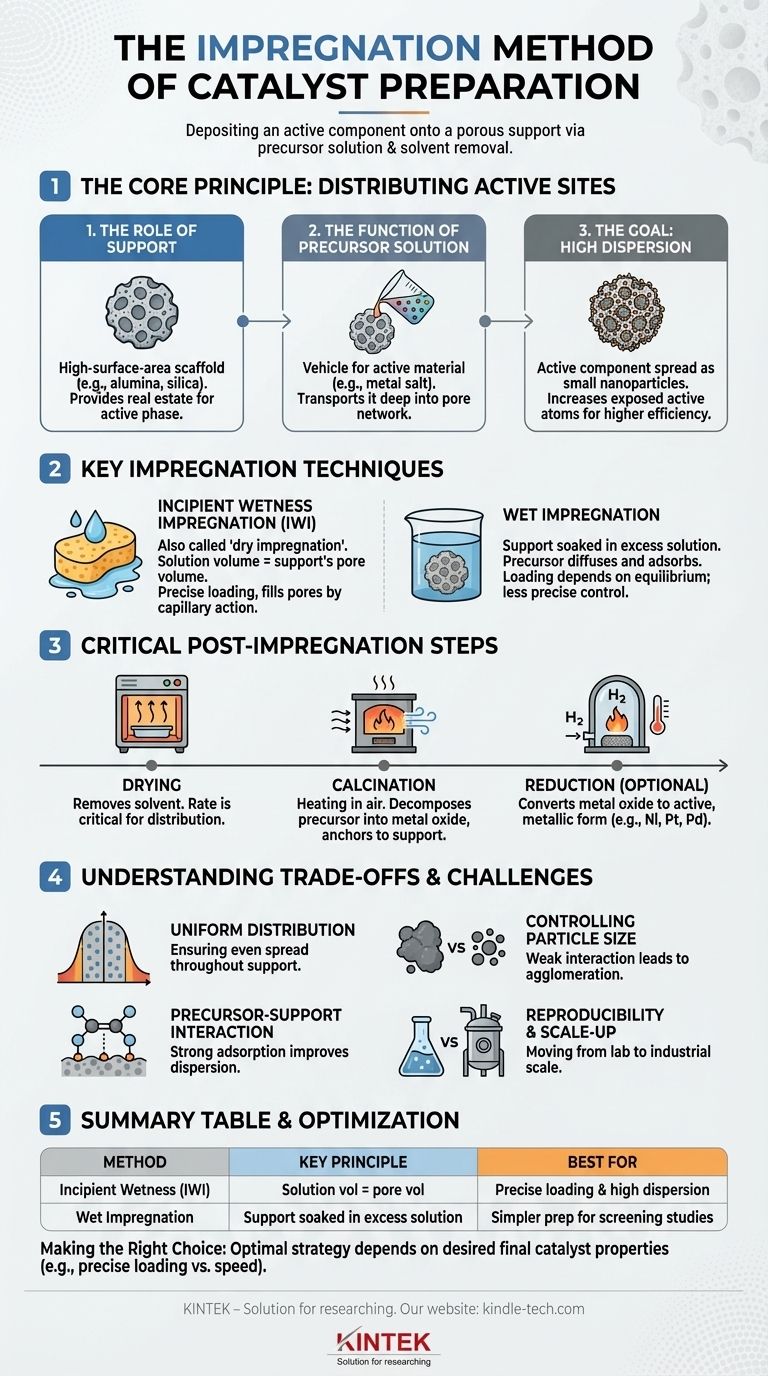
In catalyst preparation, impregnation is a method for depositing a catalytically active component onto a porous support material. This is achieved by filling the pores of the support with a solution containing a precursor—typically a dissolved metal salt—and then removing the solvent. The precursor is left behind, finely distributed across the vast internal surface area of the support, ready for subsequent conversion into its active form.
The core principle of impregnation is to leverage the high surface area of a stable support to achieve a high dispersion of the active catalytic phase. Success hinges on controlling the precursor-support interaction and the subsequent drying process to prevent the active material from clustering into ineffective, large particles.

The Core Principle: Distributing Active Sites
To understand impregnation, you must first understand its fundamental goal: creating the maximum number of active sites for a chemical reaction to occur.
The Role of the Support
The support (e.g., alumina, silica, activated carbon) is not just a passive carrier. It is a high-surface-area scaffold, often possessing hundreds of square meters of surface area per gram. This structure provides the real estate upon which the active phase is built.
The Function of the Precursor Solution
The precursor solution contains the active component in a dissolved, mobile form, such as a metal salt (e.g., nickel nitrate for a nickel catalyst). This solution is the vehicle used to transport the active material deep into the pore network of the support.
The Goal: High Dispersion
The objective is high dispersion, meaning the active component is spread out as extremely small nanoparticles rather than large clumps. A highly dispersed catalyst exposes a greater number of active atoms to the reactants, dramatically increasing the catalyst's efficiency and activity.
Key Impregnation Techniques
While the principle is simple, the execution varies. The two primary methods are defined by the amount of solution used relative to the support's capacity.
Incipient Wetness Impregnation (IWI)
Also known as dry impregnation, this is the most common technique. It involves adding a volume of precursor solution that is equal to or slightly less than the total pore volume of the support material.
The process is analogous to a sponge absorbing exactly the amount of water it can hold. All the precursor solution is drawn into the pores by capillary action, ensuring that all the dissolved metal salt is deposited within the support structure as the solvent evaporates.
Wet Impregnation
In this method, the support is submerged in an excess volume of the precursor solution. The support is allowed to soak for a period, during which the precursor diffuses into the pores and adsorbs onto the support surface.
After soaking, the excess solution is filtered off. The amount of precursor loaded onto the support depends on factors like adsorption equilibrium, concentration, and temperature, which can make precise control more challenging than with IWI.
The Critical Post-Impregnation Steps
Deposition is only the first step. The impregnated support must then be processed to create the final, active catalyst.
- Drying: This step removes the solvent (usually water). The drying rate is critical; slow drying can cause the dissolved precursor to migrate with the liquid to the exterior of the support pellet, creating an "eggshell" distribution. Rapid drying can help trap the precursor more uniformly.
- Calcination: After drying, the material is heated to a high temperature in air. This process decomposes the precursor salt into a more stable metal oxide and firmly anchors it to the support.
- Reduction: For many metallic catalysts (e.g., Ni, Pt, Pd), a final reduction step is required. The calcined oxide is exposed to a reducing gas like hydrogen at high temperatures to convert the metal oxide into the active, metallic form.
Understanding the Trade-offs and Challenges
Impregnation is a powerful technique, but it is not without complexity. The quality of the final catalyst depends on a delicate balance of chemical and physical factors.
Achieving Uniform Distribution
The primary challenge is ensuring the active phase is distributed evenly throughout the support. Poor control during impregnation or drying can lead to the active material concentrating on the external surface, which may be undesirable and is an inefficient use of expensive metals like platinum or palladium.
Controlling Metal Particle Size
The final size of the active metal particles is determined by the entire process. A weak interaction between the precursor and the support allows the precursor molecules to move and agglomerate during drying and calcination, resulting in large, less active particles.
The Precursor-Support Interaction
The chemical interaction between the dissolved metal precursor and the support surface is crucial. Strong electrostatic or chemical adsorption helps to anchor the precursor in place upon initial contact, leading to a much better final dispersion. This interaction can be manipulated by adjusting the pH of the solution or chemically modifying the support surface.
Reproducibility and Scale-Up
What works perfectly in a small laboratory beaker can be difficult to reproduce in a large industrial reactor. Ensuring that every kilogram of support material is treated identically—with uniform wetting, drying, and heat treatment—is a significant engineering challenge.
Making the Right Choice for Your Goal
The optimal impregnation strategy is dictated by the desired properties of the final catalyst.
- If your primary focus is precise metal loading and high dispersion: Incipient wetness impregnation is the superior method, as it deposits a known quantity of precursor within the support's pore network.
- If your primary focus is simplicity for a screening study: Wet impregnation can be a faster method to prepare a series of catalysts, though with less control over the final loading and distribution.
- If you need to concentrate active sites near the particle surface (an "eggshell" catalyst): Use a precursor that strongly adsorbs to the support and follow with rapid drying to minimize inward diffusion.
- If you need a uniform distribution throughout the support: Select a precursor-support system with strong interaction, use incipient wetness, and employ a carefully controlled, slow drying procedure.
Ultimately, mastering impregnation is about carefully controlling the journey of the metal precursor from a liquid solution to a highly dispersed active site on the support.
Summary Table:
| Impregnation Method | Key Principle | Best For |
|---|---|---|
| Incipient Wetness (IWI) | Solution volume equals support pore volume | Precise metal loading & high dispersion |
| Wet Impregnation | Support soaked in excess solution | Simpler preparation for screening studies |
Ready to optimize your catalyst preparation? KINTEK specializes in providing high-quality lab equipment and consumables essential for precise impregnation processes, including furnaces for calcination and reduction. Our expertise supports laboratories in achieving superior catalyst performance and reproducibility. Contact our experts today to discuss your specific needs and discover how our solutions can enhance your research and development.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What is the function of sieving equipment in CuAlMn alloys? Master Pore Size Precision
- Why is a precision vibrating sieve shaker essential for metal leaching research? Optimize Your Particle Size Analysis
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
- What is the primary function of a mechanical sieve shaker for biomass analysis? Optimize Particle Size Distribution
- What are the factors affecting sieving performance and efficiency? Optimize Your Particle Separation Process


















