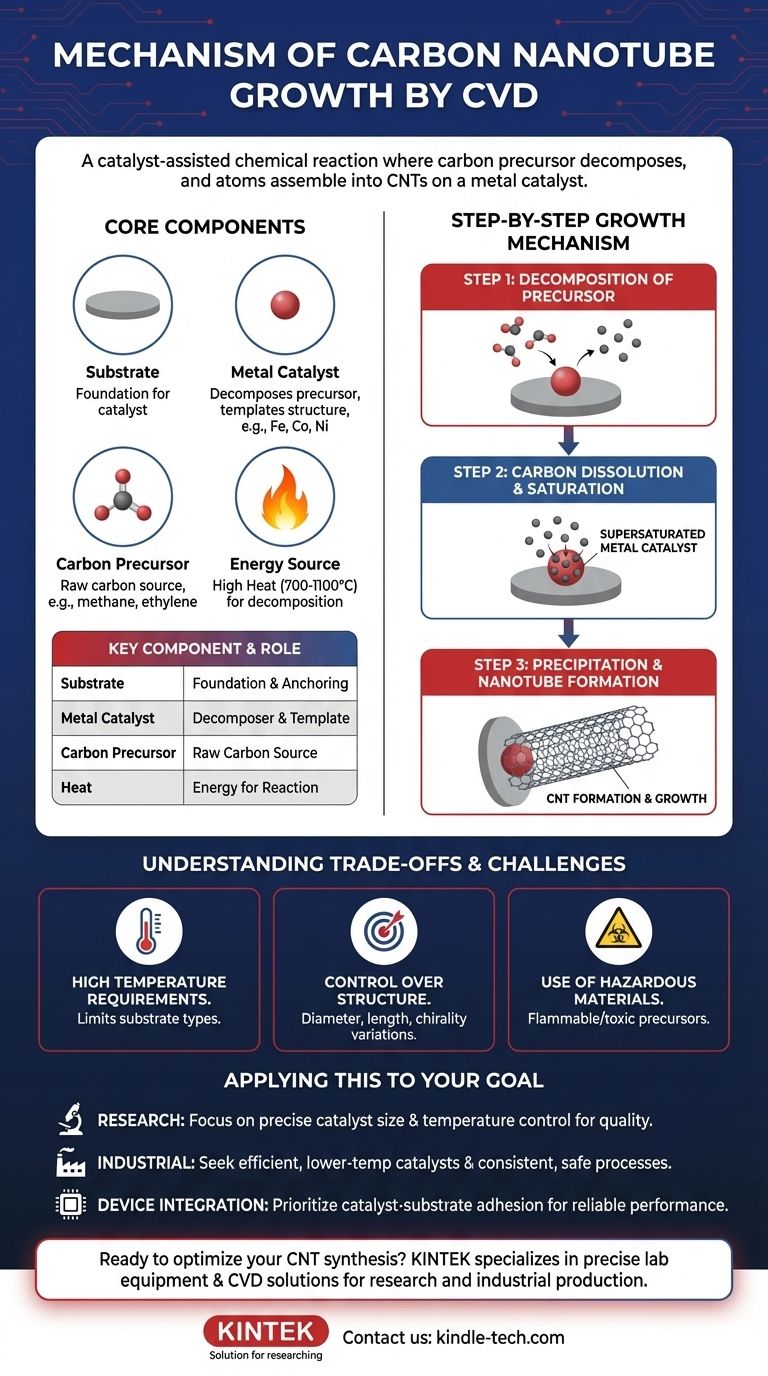
The fundamental mechanism of carbon nanotube (CNT) growth by Chemical Vapor Deposition (CVD) is a catalyst-assisted chemical reaction. In this process, a carbon-containing gas, known as a precursor, is heated until it decomposes. The resulting carbon atoms are then absorbed by nano-sized metal catalyst particles, which assemble them into the cylindrical, hexagonal lattice structure of a carbon nanotube.
At its core, CVD for nanotube synthesis is not a simple coating process. It is a controlled, high-temperature assembly line where a metal catalyst acts as both a 'cracker' for the carbon source and a 'template' for building the nanotube structure atom by atom.
The Core Components of the CVD Process
To understand the mechanism, we must first understand the role of each key component. The entire process takes place inside a reaction chamber under controlled temperature and pressure.
The Substrate
The substrate serves as the foundation for the growth process. It is typically a stable material like silicon dioxide that can withstand the high temperatures required. Its primary function is to provide a surface on which the metal catalyst can be deposited and anchored.
The Metal Catalyst
This is the most critical element in the process. A thin layer of a metal catalyst (commonly iron, cobalt, or nickel) is deposited onto the substrate. At high temperatures, this layer breaks up into tiny nanoparticles, each of which becomes a seed for the growth of a single nanotube. The catalyst's role is twofold: it dramatically lowers the energy needed to break down the precursor gas, and its size directly influences the diameter of the resulting nanotube.
The Carbon Precursor
The carbon precursor is a hydrocarbon gas (such as acetylene, ethylene, or methane) that is flowed into the reaction chamber. At high temperatures, this gas becomes unstable and is ready to release its carbon atoms. This gas is the raw material from which the nanotubes are built.
The Energy Source (Heat)
High temperature, often between 700°C and 1100°C, provides the thermal energy necessary to initiate and sustain the chemical reactions. Heat energizes the catalyst particles and facilitates the decomposition (pyrolysis) of the carbon precursor gas on the catalyst's surface.
The Step-by-Step Growth Mechanism
The growth of a carbon nanotube from these components follows a precise sequence of events at the nanoscale.
Step 1: Decomposition of the Precursor
As the precursor gas flows over the heated substrate, it comes into contact with the hot metal catalyst nanoparticles. The catalyst's surface provides an active site that efficiently breaks the chemical bonds of the gas molecules, releasing free carbon atoms.
Step 2: Carbon Dissolution and Saturation
The liberated carbon atoms diffuse, or dissolve, into the metal catalyst particle. This process continues until the nanoparticle becomes supersaturated with carbon—it has absorbed more carbon than it can hold in a stable equilibrium.
Step 3: Precipitation and Nanotube Formation
Once supersaturated, the catalyst must expel the excess carbon. The carbon atoms precipitate out from the particle, but they do so in an organized fashion, bonding together to form the stable, hexagonal graphitic lattice. This precipitation forms the cylindrical wall of the carbon nanotube, which then begins to grow outwards from the catalyst particle.
Understanding the Trade-offs and Challenges
While CVD is a powerful method for growing CNTs, it is not without its challenges. Understanding these limitations is key to successful implementation.
High Temperature Requirements
The extremely high temperatures needed can damage or limit the types of substrates that can be used. This makes it difficult to grow CNTs directly on sensitive materials like certain plastics or electronic components.
Control Over Structure
Achieving precise control over the final nanotube structure—its diameter, length, and specific atomic arrangement (chirality)—remains a significant challenge. Minor fluctuations in temperature or catalyst particle size can lead to variations in the final product.
Use of Hazardous Materials
The process often involves precursor gases and other chemicals that can be flammable, explosive, or toxic. This necessitates strict safety protocols for handling and disposal to protect both personnel and the environment.
Applying This to Your Goal
Your approach to CNT synthesis via CVD should be guided by your ultimate objective.
- If your primary focus is high-purity research: Your efforts should center on precise control of catalyst particle size and process temperature, as these variables have the most direct impact on nanotube diameter and quality.
- If your primary focus is industrial-scale production: The main goal is to find catalysts that operate efficiently at lower temperatures and to engineer a process that ensures consistent, repeatable results while managing the safe handling of precursor gases.
- If your primary focus is integrating CNTs into devices: You must prioritize the interaction between the catalyst and the substrate to ensure strong adhesion, which is critical for reliable performance in applications like electronics and sensors.
By mastering these fundamental principles, you can effectively control the synthesis of carbon nanotubes for a vast range of advanced applications.
Summary Table:
| Key Component | Role in CNT Growth |
|---|---|
| Substrate | Foundation for catalyst deposition and anchoring |
| Metal Catalyst | Decomposes precursor and templates nanotube structure |
| Carbon Precursor | Provides the raw carbon atoms for nanotube assembly |
| Heat (700-1100°C) | Supplies energy for precursor decomposition and reactions |
Ready to optimize your carbon nanotube synthesis? KINTEK specializes in providing the precise lab equipment and consumables—from CVD systems to catalyst materials—needed to achieve controlled, high-quality CNT growth for research or industrial production. Contact our experts today to discuss how our solutions can advance your materials science projects.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- What is the floating catalyst method? A Guide to High-Yield CNT Production



















