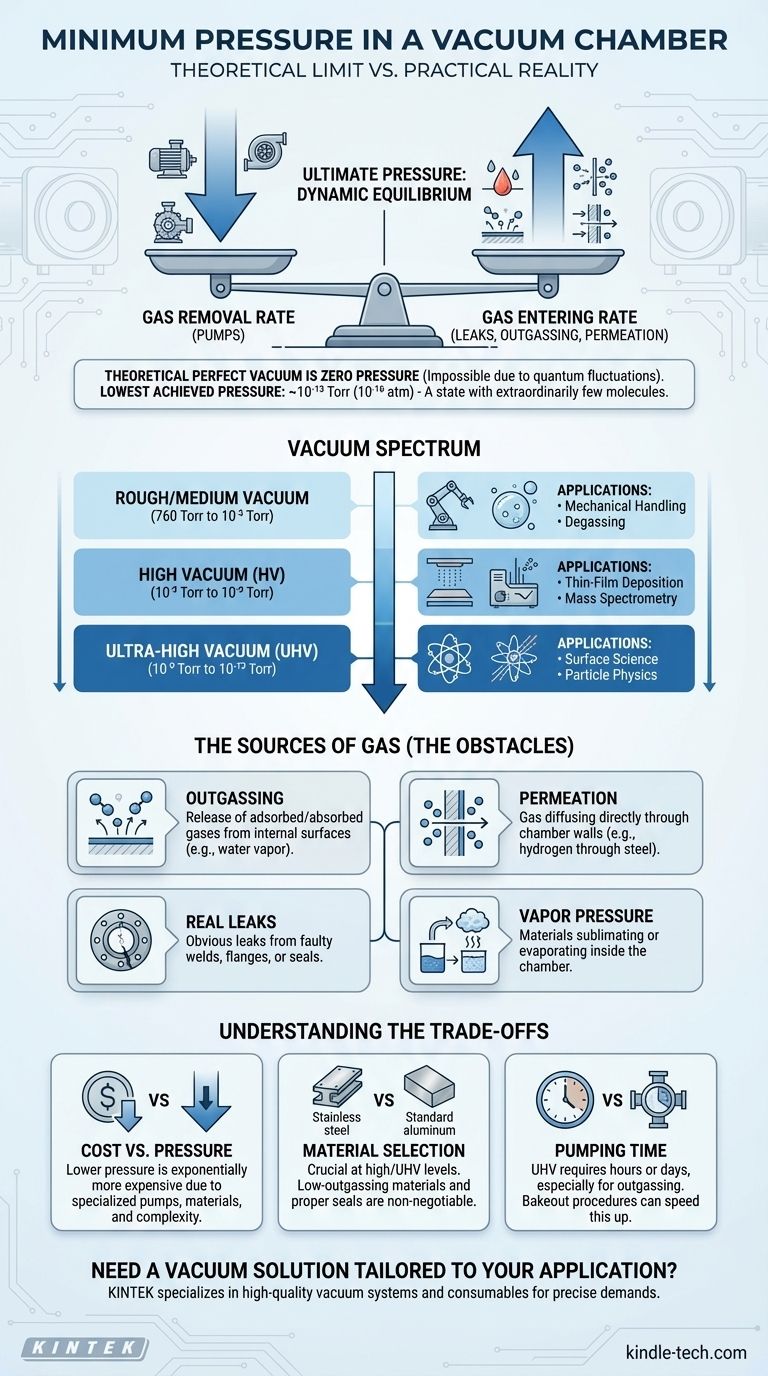
In principle, the minimum pressure in a vacuum chamber is zero, but in practice, this "perfect vacuum" is physically unattainable. The lowest pressure ever achieved in a laboratory setting is on the order of 10⁻¹³ Torr (or 10⁻¹⁶ atm), a state with extraordinarily few gas molecules remaining. The ultimate vacuum level in any system is not a static number but a dynamic equilibrium determined by the battle between pumping gas out and new gas entering the system.
The core concept to understand is that the minimum pressure in any vacuum chamber is the point at which the rate of gas removal by the pumps is exactly equal to the rate of gas entering the chamber from leaks, material outgassing, and permeation.

What "Vacuum" Actually Means
A vacuum is fundamentally a space devoid of matter. However, creating a space with truly zero atoms, molecules, or particles is impossible. The quality of a vacuum is therefore defined by how closely it approaches this ideal state, measured by its residual gas pressure.
The Theoretical Limit of a Perfect Vacuum
Even if a chamber could be made perfectly sealed and all matter removed, it would not be truly empty. According to quantum mechanics, the vacuum of space is filled with constantly fluctuating quantum fields, giving rise to "virtual particles" that pop in and out of existence. This represents a fundamental floor below which pressure cannot exist.
The Practical Limits of Real-World Systems
In any real-world vacuum chamber, the practical limit is set by the introduction of gas molecules. The final pressure, often called the ultimate pressure, is reached when the pumping system can no longer reduce the pressure further because its removal rate is matched by the rate of gas entering the system.
The Sources of Gas in a Vacuum System
Achieving lower pressures is a constant fight against gas molecules entering the vacuum space. These molecules come from several persistent sources that become increasingly significant as the pressure drops.
Outgassing: The Primary Obstacle
Outgassing is the release of adsorbed or absorbed gases from the internal surfaces of the vacuum chamber and its components. Water vapor is the most common outgassing species, clinging tightly to surfaces. This is why high-vacuum systems are often "baked out"—heated to hundreds of degrees to drive off this water and other trapped gases.
Permeation: Gas Through Solid Barriers
Permeation is the process where gas molecules from the outside atmosphere diffuse directly through the solid walls of the chamber. Lighter gases like hydrogen and helium are particularly prone to permeating through materials, including stainless steel and elastomeric seals like Viton.
Real Leaks: The Obvious Culprit
Obvious leaks from faulty welds, flanges, or seals can prevent a system from reaching low pressure. While critical to address, these are often less of a challenge in ultra-high vacuum (UHV) systems than the more subtle effects of outgassing and permeation.
Vapor Pressure: When Solids and Liquids Become Gas
Every material has a vapor pressure, meaning it will sublimate (solid to gas) or evaporate (liquid to gas) to some degree. This is why materials inside a vacuum chamber must be carefully selected. Materials with high vapor pressures, like certain plastics, oils, or even metals like zinc and cadmium, will continuously create gas and limit the ultimate pressure.
Understanding the Trade-offs
Designing a vacuum system involves balancing performance requirements with practical constraints. The pursuit of lower pressure comes with significant trade-offs.
Cost vs. Ultimate Pressure
Achieving progressively lower pressures is exponentially more expensive. A simple rough vacuum system may cost a few thousand dollars, while an ultra-high vacuum system for surface science research can easily cost hundreds of thousands. This is due to the need for multiple, specialized pumps (turbomolecular, ion, cryogenic), exotic materials, and complex bakeout procedures.
Material Selection is Non-Negotiable
At high and ultra-high vacuum levels, material choice is paramount. Standard materials like aluminum are more porous and have higher outgassing rates than vacuum-fired stainless steel. Using the wrong elastomer seal or a component with a high vapor pressure can make it impossible to reach the desired pressure, regardless of pumping power.
Time is a Factor
Pumping a chamber down to UHV levels is not instantaneous. The process can take many hours or even days. The majority of this time is spent waiting for the outgassing rate from the chamber walls to slowly decrease. A bakeout procedure can dramatically speed this up but adds complexity to the system.
Making the Right Choice for Your Goal
The "minimum pressure" you need is dictated entirely by your application. Defining your goal is the first step to specifying the right system.
- If your primary focus is mechanical handling or degassing (Rough/Medium Vacuum): Your main concern is removing the bulk atmosphere, so a simple mechanical pump and standard materials are sufficient.
- If your primary focus is thin-film deposition or operating a mass spectrometer (High Vacuum): You need a multi-stage pumping system (e.g., roughing pump + turbopump) and must use clean, low-outgassing materials like stainless steel.
- If your primary focus is surface science or particle physics research (Ultra-High Vacuum): Your system requires an all-metal construction, extensive bakeout capabilities, and specialized UHV pumps to overcome the fundamental limits of outgassing and permeation.
Ultimately, the minimum achievable pressure is not a universal constant but a meticulously engineered equilibrium specific to each vacuum system.
Summary Table:
| Vacuum Level | Typical Pressure Range | Key Applications | Primary Gas Sources |
|---|---|---|---|
| Rough/Medium Vacuum | 760 Torr to 10⁻³ Torr | Mechanical Handling, Degassing | Bulk Atmosphere, Real Leaks |
| High Vacuum (HV) | 10⁻³ Torr to 10⁻⁹ Torr | Thin-Film Deposition, Mass Spectrometry | Outgassing, Vapor Pressure |
| Ultra-High Vacuum (UHV) | 10⁻⁹ Torr to 10⁻¹³ Torr | Surface Science, Particle Physics | Permeation, Residual Outgassing |
Need a Vacuum Solution Tailored to Your Application?
Whether you're working in thin-film deposition, surface science, or general laboratory processing, achieving the right vacuum pressure is critical to your success. The team at KINTEK specializes in providing high-quality lab equipment, including vacuum systems and consumables, designed to meet the precise demands of your research or production environment.
We understand that the 'minimum pressure' isn't just a number—it's the key to your experiment's integrity and efficiency. Let us help you navigate the trade-offs between cost, materials, and performance to specify the ideal system for your goals.
Contact us today using the form below to discuss your vacuum requirements and discover how KINTEK can support your laboratory's needs.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Laboratory Rotary Vane Vacuum Pump for Lab Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
People Also Ask
- What is the significance of using high-vacuum heat treatment furnaces and rapid quenching for zirconium alloys?
- Why are strictly controlled melting and processing systems required for FeCrAl? Ensure Peak Material Integrity
- What is three step sintering process? A Guide to Blending, Compacting, and Heating
- What are the parts of a vacuum furnace? A Guide to the 5 Core Systems
- What are two advantages of putting sinter into the furnace? Achieve Purity and Superior Strength
- What are the different annealing techniques? A Guide to Softening, Stress Relief, and Machinability
- How does a vacuum drying oven benefit Al2O3-TiCN/Co-Ni slurry processing? Protect Material Integrity & Purity
- What role do high-precision melting furnaces play in stir casting? Master Precision in Zinc-Based Composites



















