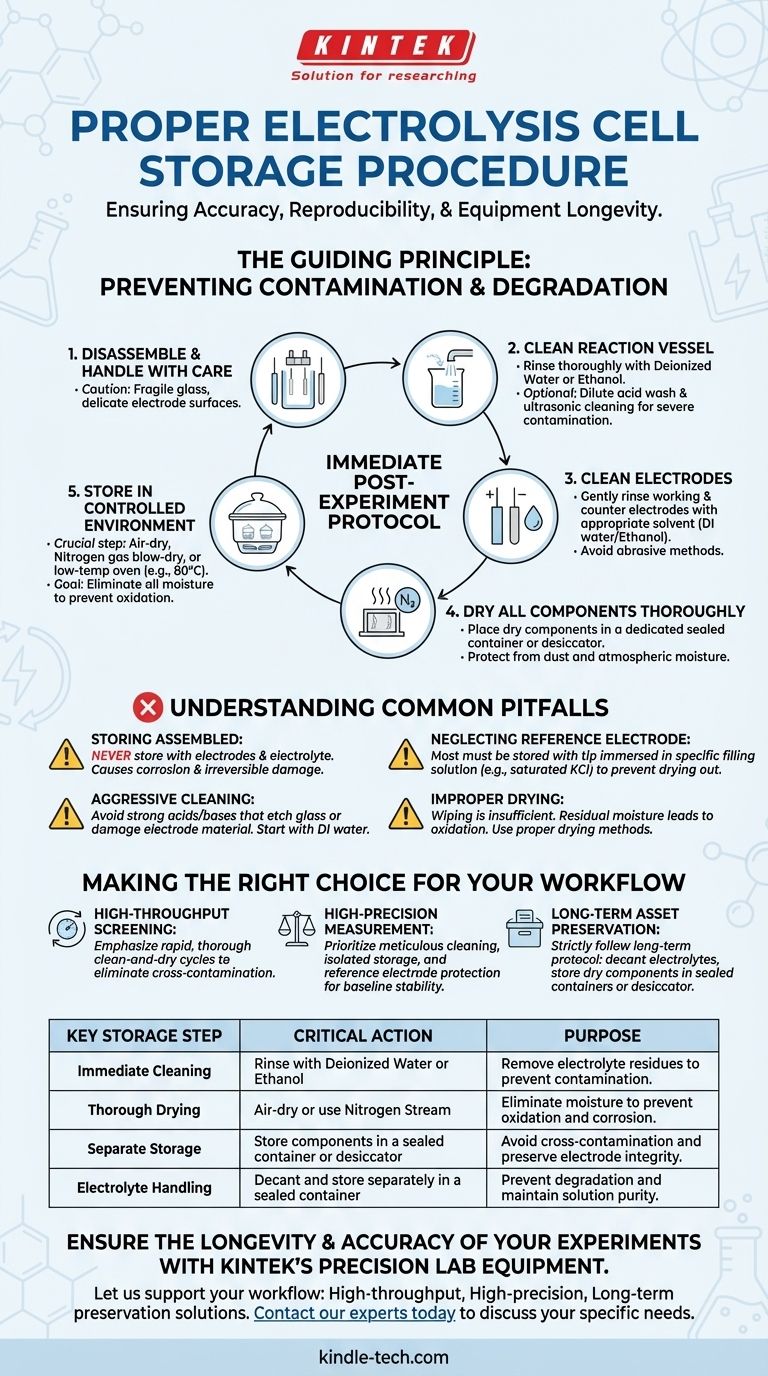
To properly store an electrolysis cell, you must follow a strict post-experiment protocol of immediate cleaning, thorough drying, and careful, separate storage of its components. After disassembling the cell, clean the vessel and electrodes with deionized water or ethanol, dry them completely, and store them in a dry, dust-free environment. For any long-term storage, the electrolyte must be decanted and stored separately in a sealed container.
Improper storage is not a matter of tidiness; it is a direct threat to the accuracy and reproducibility of your electrochemical measurements. The core objective of any storage procedure is to prevent contamination and material degradation, thereby preserving the integrity of your sensitive equipment.

The Guiding Principle: Preventing Contamination and Degradation
The entire storage process is built on one foundational concept: an electrolysis cell is a high-precision instrument. Its surfaces and solutions must remain in a known, pristine state to produce reliable data.
Why Immediate Cleaning is Non-Negotiable
Residues from your electrolyte or reaction byproducts must be removed immediately after an experiment. If left to dry, these residues can adhere strongly to glass and electrode surfaces, becoming difficult to remove and creating a source of contamination for future experiments.
The Enemy: Moisture and Corrosion
Moisture is a primary cause of electrode degradation. Even trace amounts of water, especially when combined with ionic residue, can lead to corrosion or oxidation on the electrode surface. A thoroughly dry environment is critical for preservation.
Preserving Component Integrity
Storing components separately is essential. The reaction vessel, working electrode, counter electrode, and reference electrode each have unique materials and storage needs. Storing them assembled allows for potential cross-contamination and the degradation of sensitive parts, like a reference electrode drying out.
A Step-by-Step Post-Experiment Protocol
Follow this procedure diligently after every experiment to ensure the longevity and accuracy of your equipment.
Step 1: Disassemble and Handle with Care
Once your experiment is complete, carefully disassemble the cell. Remember that glass components are fragile and electrode surfaces can be scratched easily.
Step 2: Clean the Reaction Vessel
Rinse the cell body thoroughly with deionized water. For organic electrolytes or stubborn residues, a rinse with ethanol may be necessary. For deep cleaning, a dilute acid wash (like 5% nitric acid) followed by ultrasonic cleaning in deionized water can be used, but this is typically reserved for new cells or severe contamination.
Step 3: Clean the Working and Counter Electrodes
Gently rinse the working and counter electrodes with deionized water or the appropriate solvent to remove all traces of electrolyte. Avoid abrasive cleaning methods that could alter the electrode's surface area or structure.
Step 4: Dry All Components Thoroughly
Drying is a critical step. You can allow components to air-dry in a dust-free area, gently blow-dry them with a stream of nitrogen gas, or place them in a low-temperature oven (e.g., 80°C) for a short period. The goal is to remove all moisture without thermally damaging the components.
Step 5: Store in a Controlled Environment
Place the clean, dry electrodes and cell body in a dedicated, sealed container or a desiccator. This protects them from atmospheric moisture and dust, ensuring they are ready for the next use.
Understanding the Common Pitfalls
Avoiding common mistakes is as important as following the correct procedure. Mishandling your equipment can quickly lead to costly damage and unreliable data.
The Critical Mistake of Storing the Cell Assembled
Never store the cell with the electrodes and electrolyte still inside. This is the fastest way to cause corrosion, electrode fouling, and irreversible damage to your reference electrode.
Neglecting the Reference Electrode
A common and costly error is allowing a reference electrode to dry out. Most reference electrodes must be stored with their tip immersed in a specific filling solution (often a saturated KCl solution) to maintain their electrochemical potential. Letting them dry out can permanently damage them.
Aggressive Cleaning vs. Electrode Damage
While cleaning is essential, using overly aggressive chemicals can be destructive. Strong acids or bases can etch glass or, more importantly, damage the electrode material itself. Always start with the mildest effective solvent—deionized water—before escalating.
Improper Drying Leads to Oxidation
Simply wiping a component "dry" is insufficient. Residual microscopic water droplets can still cause oxidation. A proper drying process, like using a nitrogen stream or a low-temperature oven, ensures all moisture is truly eliminated.
Making the Right Choice for Your Workflow
Your specific experimental goals will influence how you apply these principles.
- If your primary focus is high-throughput screening: Emphasize a rapid but thorough clean-and-dry cycle between runs to eliminate the risk of cross-contamination, which is paramount.
- If your primary focus is high-precision measurement: Prioritize meticulous cleaning and isolated storage of each component, paying special attention to protecting the reference electrode to ensure baseline stability.
- If your primary focus is long-term asset preservation: Strictly follow the long-term storage protocol, including decanting electrolytes and placing all dry components in sealed, dedicated containers or a desiccator.
By treating post-experiment storage as an integral part of the experiment itself, you guarantee the long-term reliability of your equipment and the integrity of your results.
Summary Table:
| Key Storage Step | Critical Action | Purpose |
|---|---|---|
| Immediate Cleaning | Rinse with deionized water or ethanol | Remove electrolyte residues to prevent contamination |
| Thorough Drying | Air-dry or use nitrogen stream | Eliminate moisture to prevent oxidation and corrosion |
| Separate Storage | Store components in a sealed container or desiccator | Avoid cross-contamination and preserve electrode integrity |
| Electrolyte Handling | Decant and store separately in a sealed container | Prevent degradation and maintain solution purity |
Ensure the longevity and accuracy of your electrochemical experiments with KINTEK's precision lab equipment.
Proper storage is just one part of achieving reliable results. KINTEK specializes in high-quality electrolysis cells, durable electrodes, and essential lab consumables designed for researchers who demand precision and reproducibility.
Let us support your workflow:
- High-throughput screening? Our easy-to-clean cell designs minimize cross-contamination risk.
- High-precision measurements? We offer stable reference electrodes and inert cell materials.
- Long-term asset preservation? Our equipment is built for durability and comes with storage guidelines.
Contact our experts today to discuss your specific laboratory needs and discover how KINTEK's solutions can enhance your electrochemical research.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained



















