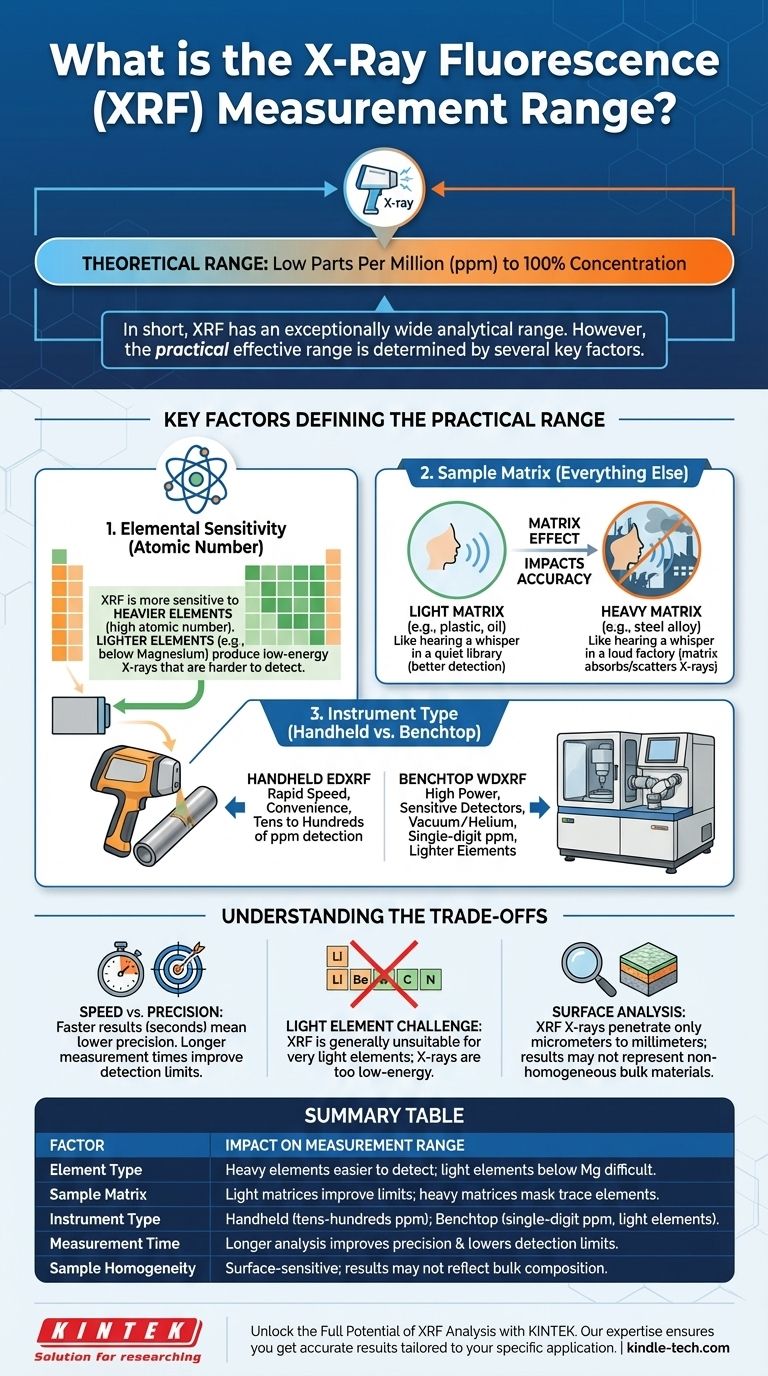
In short, X-Ray Fluorescence (XRF) has an exceptionally wide analytical range, capable of measuring elemental concentrations from low parts per million (ppm) up to 100%. However, this range is not universal for all elements or all sample types. The practical, effective range for your specific application is determined by the element being measured, the surrounding material, and the type of instrument you are using.
The core takeaway is that while XRF is powerful and versatile, its performance is not a single, fixed number. Understanding the interplay between the element of interest, the sample matrix, and the instrument's design is critical to determining if it is the right tool for your specific measurement needs.

The Fundamentals: How XRF Determines Range
X-ray Fluorescence operates on a simple principle. The instrument bombards a sample with high-energy X-rays, which excites the atoms within. These atoms then release their own secondary, "fluorescent" X-rays, each with a characteristic energy signature that acts as a fingerprint for a specific element.
Intensity Equals Concentration
The instrument's detector counts the number of these characteristic X-rays. In general, a higher intensity of a specific X-ray signature means there is a higher concentration of that element in the sample. The measurement "range" is simply the span between the lowest concentration the instrument can reliably detect and the highest concentration it can measure (which is typically 100%).
Key Factors Defining the Measurement Range
The broad "ppm to 100%" range is a theoretical maximum. In practice, three primary factors dictate the achievable limits for any given analysis.
Elemental Sensitivity (Atomic Number)
XRF is significantly more sensitive to heavier elements (those with a high atomic number, like lead or gold) than it is to light elements (like sodium or aluminum).
Heavier elements produce higher-energy fluorescent X-rays that are easier for the detector to "see." Lighter elements produce low-energy X-rays that are often re-absorbed within the sample or blocked by air, making them much harder to detect. For most standard XRF systems, elements lighter than magnesium (Mg) are very difficult or impossible to measure.
The Role of the Sample Matrix
The "matrix" is everything in the sample that is not the element you are trying to measure. This matrix has a profound impact on the accuracy and detection limits of the analysis.
This is known as a matrix effect. Imagine trying to hear a whisper. In a quiet library (a light matrix like plastic or oil), you can hear it easily. In a loud factory (a heavy matrix like a steel alloy), that same whisper is completely drowned out. The matrix can absorb or scatter the X-rays, preventing them from reaching the detector and leading to an under-reported result.
Instrument Type: Handheld vs. Benchtop
The physical instrument itself is a major factor. There are two main categories: portable Energy Dispersive XRF (EDXRF) and laboratory-grade Wavelength Dispersive XRF (WDXRF).
- Handheld EDXRF: These portable units are designed for speed and convenience. They are excellent for identifying alloys and screening materials for restricted substances, typically with detection limits in the tens or hundreds of ppm for most elements.
- Benchtop WDXRF: These are larger, more powerful laboratory systems. They use higher-power X-ray tubes, more sensitive detectors, and often a vacuum or helium environment to measure much lower concentrations (down to single-digit ppm) and lighter elements.
Understanding the Trade-offs
Choosing XRF requires acknowledging its practical limitations. It is not a perfect solution for every analytical problem.
Speed vs. Precision
XRF is incredibly fast, often providing a result in seconds. However, achieving the lowest possible detection limits requires longer measurement times. A 5-second test might be sufficient for alloy identification, but a 5-minute test might be needed to confirm a trace contaminant is below a regulatory threshold.
The Challenge of Light Elements
It is critical to reiterate that XRF is generally unsuitable for measuring very light elements like carbon, lithium, beryllium, or boron. The low-energy X-rays these elements produce simply do not escape the sample to reach the detector in sufficient quantities.
Surface vs. Bulk Analysis
This is perhaps the most common pitfall for new users. XRF is a surface analysis technique. The X-rays only penetrate a small distance into the material—from a few micrometers to several millimeters, depending on the sample's density.
If your sample is not homogenous (for example, a plated piece of metal or a contaminated soil particle), the XRF result will only represent the surface composition, which may not be representative of the bulk material.
Making the Right Choice for Your Goal
To determine if XRF's range fits your needs, consider your primary objective.
- If your primary focus is rapid material identification or sorting: A handheld XRF is an ideal tool. Its ability to distinguish between percent-level concentrations in seconds is its greatest strength.
- If your primary focus is precise compliance testing or trace element analysis: A benchtop XRF is likely necessary, and you must carefully manage matrix effects and use longer measurement times to achieve low ppm-level detection limits.
- If your primary focus is measuring light elements or requiring sub-ppm accuracy: XRF is likely the wrong technique. You should explore alternatives like Inductively Coupled Plasma (ICP) or Optical Emission Spectrometry (OES).
Ultimately, harnessing the power of XRF begins with understanding that its effective range is a direct consequence of your specific analytical problem.
Summary Table:
| Factor | Impact on Measurement Range |
|---|---|
| Element Type | Heavier elements (high atomic number) are easier to detect than lighter elements (e.g., below magnesium). |
| Sample Matrix | Light matrices (e.g., plastics) offer better detection limits; heavy matrices (e.g., alloys) can mask trace elements. |
| Instrument Type | Handheld EDXRF: tens to hundreds of ppm; Benchtop WDXRF: single-digit ppm and lighter elements. |
| Measurement Time | Longer analysis times improve precision and lower detection limits. |
| Sample Homogeneity | XRF is surface-sensitive; results may not represent bulk composition for non-uniform samples. |
Unlock the Full Potential of XRF Analysis with KINTEK
Are you navigating the complexities of elemental analysis? Whether you need rapid material identification with a handheld XRF or precise, trace-level detection with a benchtop system, KINTEK has the right solution for your laboratory. Our expertise in lab equipment ensures you get accurate, reliable results tailored to your specific application—from alloy sorting to compliance testing.
Let us help you optimize your analytical workflow. Contact our experts today to discuss your needs and discover how KINTEK's XRF solutions can enhance your lab's capabilities!
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Three-dimensional electromagnetic sieving instrument
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Engineering Advanced Fine Ceramics Head Tweezers with Pointed Elbow Zirconia Ceramic Tip
People Also Ask
- What can XRF identify? Discover the Elements in Your Materials with Precision
- Why is a laboratory hydraulic press essential for Cu-Mo alloy production? Maximize Green Body Strength and Density
- Why is a Laboratory Hydraulic Press Necessary for Composite Membrane Development? Ensure AnMBR Structural Integrity
- How are samples prepared for XRF analysis? Achieve Accurate and Reliable Results
- What is a hydraulic press for sample preparation? Create Consistent Pellets for Reliable Analysis
- What are the applications of a hydraulic press? From Metal Shaping to Material Testing
- What are the safety precautions required when using the hydraulic press? Ensure Operator and Machine Protection
- What is the function of a high-pressure hydraulic press? Optimize Silicide Bulk Material Preparation














