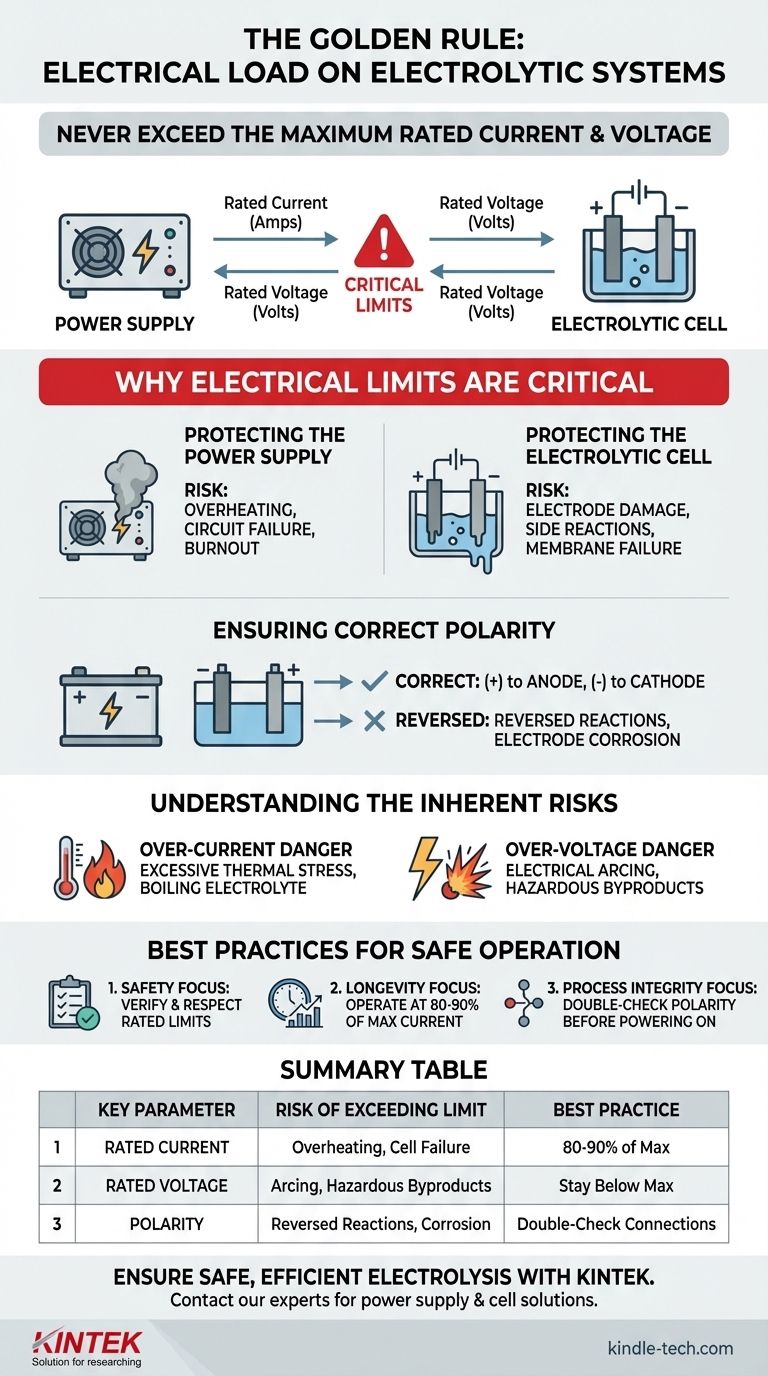
The foundational rule is to never operate an electrolytic system beyond the specified electrical limits of its components. This means you must never exceed the maximum rated current (amperage) and rated voltage for both the electrolytic cell and the power supply providing the energy. Adherence to this principle is the primary defense against equipment damage and serious safety incidents.
The specified current and voltage ratings are not suggestions; they are hard operational limits defined by the physical and chemical constraints of your equipment. Exceeding them risks catastrophic failure of the power supply, irreversible damage to the electrolytic cell, and the creation of significant safety hazards.

Why These Electrical Limits Are Critical
Understanding the role of each component clarifies why these limits are non-negotiable. The power supply and the electrolytic cell form a symbiotic system, where the weakness of one can destroy both.
Protecting the Power Supply
A power supply is engineered to deliver a specific maximum current and voltage. Pushing it beyond this design limit forces its internal components to handle more power than they were built for.
This overload condition can lead to rapid overheating, the failure of internal circuits, and in some cases, a complete and permanent burnout of the unit.
Protecting the Electrolytic Cell
The electrolytic cell itself has strict operational boundaries. The electrodes, membranes, and even the electrolyte solution have limits on the electrical energy they can safely manage.
Excessive current can cause the electrodes to overheat, degrade, or even melt. Excessive voltage can cause unwanted side reactions, break down the electrolyte, or damage sensitive components like ion-exchange membranes.
Ensuring Correct Polarity
Beyond current and voltage, the polarity of the connection is crucial. The chemical reactions of electrolysis are specific to the anode (positive terminal) and the cathode (negative terminal).
Reversing these connections will reverse the intended reactions. This can permanently damage the electrodes—for example, by corroding an electrode that was meant to be inert—and will fail to produce the desired chemical product.
Understanding the Inherent Risks
Ignoring these electrical parameters is not a shortcut; it's a direct path to failure. The consequences range from inefficient operation to dangerous and costly damage.
The Danger of Over-Current
Current is directly related to the rate of the chemical reaction, but it also generates heat (I²R losses). An over-current condition creates excessive thermal stress.
This heat can boil the electrolyte, damage seals, and warp the cell's physical structure. It is the most common cause of premature equipment failure.
The Danger of Over-Voltage
Voltage is the electrical "pressure" driving the reaction. Applying a voltage higher than the cell's rated limit can cause electrical arcing or the breakdown of the electrolyte itself.
This can lead to the production of unintended, and potentially hazardous, gases or byproducts, compromising both the process and the safety of the operating environment.
Best Practices for Safe Operation
To ensure a safe and effective process, you must treat the electrical specifications as absolute laws for your system.
- If your primary focus is safety: Always verify and respect the maximum rated current and voltage on the nameplates of both the power supply and the cell before operation.
- If your primary focus is equipment longevity: Operate your system at 80-90% of its maximum rated current to minimize thermal stress and significantly extend the life of your components.
- If your primary focus is process integrity: Before powering on, always double-check that the power supply's positive terminal is connected to the cell's anode and the negative terminal is connected to the cathode.
By treating these electrical limits as fundamental properties of your system, you ensure safe, reliable, and predictable results.
Summary Table:
| Key Electrical Parameter | Risk of Exceeding Limit | Best Practice |
|---|---|---|
| Rated Current (Amperage) | Overheating, electrode degradation, cell failure | Operate at 80-90% of max rating for longevity |
| Rated Voltage | Electrical arcing, electrolyte breakdown, hazardous byproducts | Always stay below the specified maximum voltage |
| Polarity (Anode/Cathode) | Reversed reactions, electrode corrosion, process failure | Double-check positive/negative connections before powering on |
Ensure your electrolysis processes are safe, efficient, and reliable with KINTEK.
Our specialized lab equipment and consumables are designed to meet the precise electrical demands of your electrolytic cells, helping you maintain strict adherence to current and voltage limits. Protect your investment, ensure process integrity, and achieve predictable results every time.
Contact our experts today to find the perfect power supply and cell solutions for your laboratory's unique needs.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Optical Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- How can the electrochemical reaction be controlled when using this electrolytic cell? Master Voltage, Current & Electrolyte
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety



















