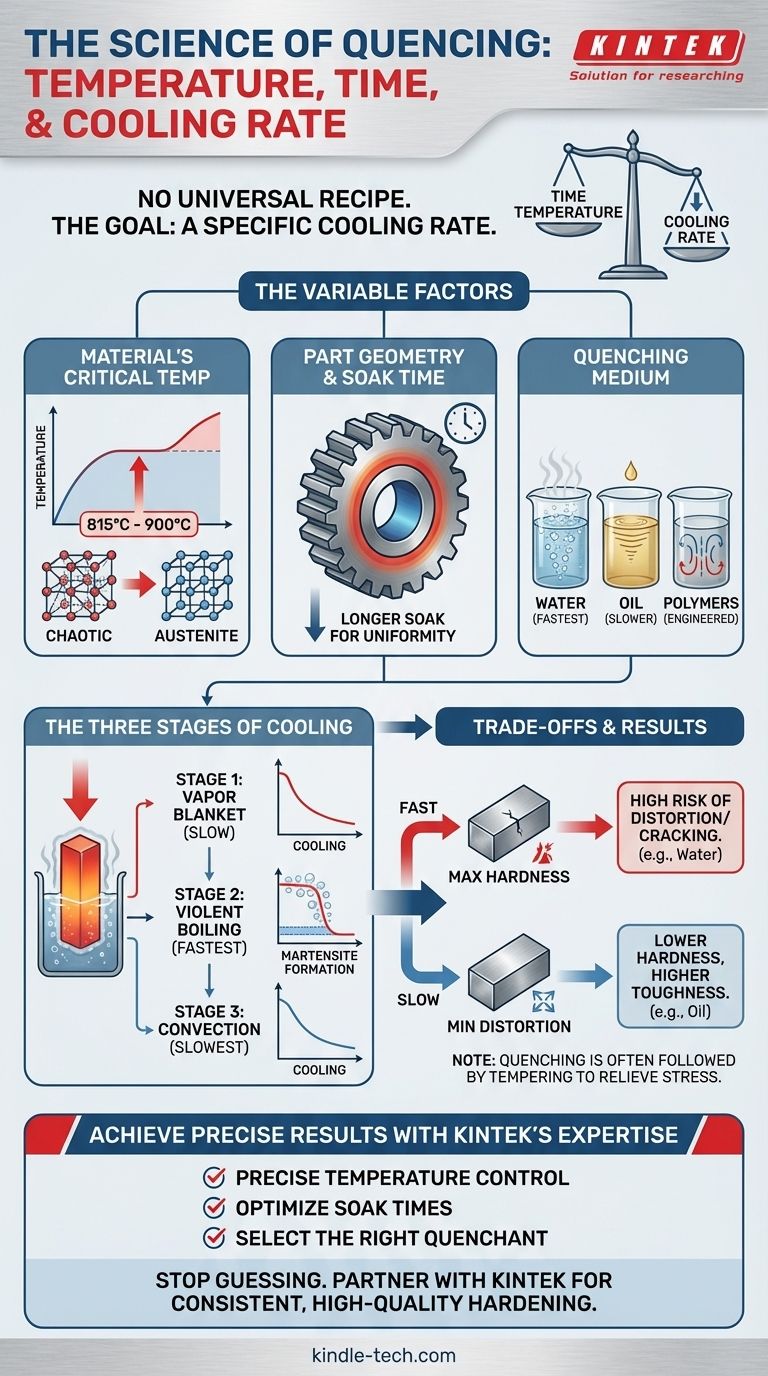
There is no single universal temperature and time for quenching. These parameters are critically dependent on the specific type of metal, the thickness of the part, and the desired final properties. The process always begins by heating the material to its austenitizing temperature, which for most steels is typically between 815°C and 900°C (1500°F and 1650°F), holding it just long enough to ensure a complete and uniform crystal structure transformation.
The core principle of quenching is not to meet a specific time, but to achieve a specific cooling rate. The goal is to cool the metal fast enough to bypass softer transformations and form a very hard crystal structure, known as martensite.

Why Quenching Parameters Are So Variable
Achieving the desired outcome from quenching requires a careful balance of three primary factors. A change in any one of these will alter the final properties of the material, such as its hardness, toughness, and internal stress.
The Material's Critical Temperatures
Every alloy has a unique "austenitizing" temperature. This is the temperature at which its internal crystal structure transforms into a phase called austenite, which is necessary for hardening.
Heating below this temperature will result in incomplete hardening. Overheating can cause grain growth, making the final product brittle. The specific alloy's phase diagram is the definitive source for this critical temperature.
The Impact of Part Geometry
The thickness and complexity of the component are crucial. A thick section cools much more slowly at its core than on its surface.
This is why the "soak time"—the duration the part is held at the austenitizing temperature—is so important. A thicker part requires a longer soak time to ensure the core reaches the full transformation temperature.
The Choice of Quenching Medium
The substance used to cool the part, known as the quenchant, dictates the cooling rate. Water provides a very fast, aggressive quench, while oil is slower and less severe. Specialized polymer quenchants can be engineered to have cooling rates between water and oil.
The choice of quenchant is determined by the material's "hardenability." Low-alloy steels like 1095 require a very fast quench (water or brine), while high-alloy steels like 4140 can be hardened with a much slower quench (oil).
The Three Stages of Cooling
Regardless of the quenchant, the cooling process occurs in three distinct stages, as heat is extracted from the metal part. Understanding these stages explains why different liquids produce different results.
The Vapor Stage
Immediately upon immersion, the hot component vaporizes the surrounding quenchant, forming a "vapor blanket." This blanket acts as an insulator, and cooling is relatively slow.
The Boiling Stage
As the surface cools slightly, the vapor blanket collapses, and the liquid quenchant makes direct contact. This initiates violent boiling, which is the stage of fastest heat transfer. It is in this phase that the cooling rate must be fast enough to form martensite.
The Convection Stage
Once the component's surface temperature drops below the quenchant's boiling point, boiling ceases. Heat is then removed via convection and conduction. This is the slowest stage of cooling. The quenchant's viscosity plays a major role here, as described in the reference regarding oil.
Understanding the Trade-offs
Quenching is not a process without risks. The extreme temperature changes induce significant stress in the material, and managing this is key to a successful outcome.
The Risk of Cracking and Distortion
Very rapid cooling, especially in complex shapes or parts with both thick and thin sections, can cause the part to warp, distort, or even crack.
The goal is to cool just fast enough to achieve the desired hardness without building up enough internal stress to cause failure. This is often why oil is chosen over water for more sensitive alloy steels.
Sacrificing Toughness for Hardness
The martensitic structure formed during a successful quench is extremely hard but also very brittle. This is why quenching is almost always followed by a secondary heat treatment process called tempering.
Tempering involves reheating the part to a much lower temperature to relieve stress and restore some toughness, albeit at the cost of a small amount of hardness.
Making the Right Choice for Your Goal
Selecting the correct quenching parameters is about matching the process to your material and your desired outcome.
- If your primary focus is maximum hardness in a simple carbon steel: You will likely use a very fast quenchant like water or brine, accepting the higher risk of distortion.
- If your primary focus is minimizing distortion in an alloy steel: You will select a slower quenchant like oil and ensure the part is tempered immediately after quenching to reduce the risk of cracking.
- If you are working with a thick or geometrically complex part: You must prioritize a sufficient soak time at the austenitizing temperature and consider a less aggressive quenchant to manage internal stress.
Ultimately, successful quenching comes from understanding the properties of your specific material and controlling the rate of cooling to achieve a precise metallurgical transformation.
Summary Table:
| Factor | Key Consideration | Typical Range/Example |
|---|---|---|
| Austenitizing Temperature | Specific to the metal alloy | 815°C - 900°C (1500°F - 1650°F) for most steels |
| Soak Time | Depends on part thickness | Longer for thicker sections to ensure uniform heat |
| Quenching Medium | Dictates cooling rate; chosen based on hardenability | Water (fastest), Oil (slower), Polymers (engineered rates) |
| Goal | Balances hardness with risk of distortion/cracking | Max hardness (water) vs. minimal distortion (oil) |
Achieve Perfect Hardening Results with KINTEK's Expertise
Quenching is a delicate balance of temperature, time, and cooling rate. Getting it wrong can lead to cracked, warped, or brittle parts. Let KINTEK's deep knowledge of thermal processing and high-quality lab equipment guide you to success.
We provide the reliable furnaces and expert support you need to:
- Precisely control austenitizing temperatures for your specific alloy.
- Determine optimal soak times for uniform transformation, even in complex geometries.
- Select the right quenchant to achieve the desired cooling rate and minimize stress.
Stop guessing and start achieving consistent, high-quality results. Contact our thermal processing specialists today to discuss your application and how KINTEK can be your partner in precision hardening.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
People Also Ask
- What is the process of annealing tubes? Achieve Optimal Softness and Ductility for Your Tubing
- What temperature is tube annealing? A Guide to Material-Specific Ranges for Optimal Results
- What is a vertical tube furnace? Leverage Gravity for Superior Uniformity and Process Control
- What is quartz tube heating? Achieve Instant, Targeted Heat with Infrared Radiation
- How do you clean a tubular furnace tube? A Step-by-Step Guide to Safe and Effective Maintenance



















