As a general principle, the sintering temperature for a metal is typically set above 60% of its absolute melting temperature (Tm). This is not a single fixed value but a range that depends entirely on the specific metal or alloy. The high temperature is essential to provide enough thermal energy for atoms to diffuse across the boundaries of the metal powder particles, fusing them into a solid mass without melting the material.
The core concept of sintering is not to melt the metal, but to heat it just enough to activate atomic movement. This solid-state diffusion process reduces the space between particles, bonding them together and increasing the density of the final component.
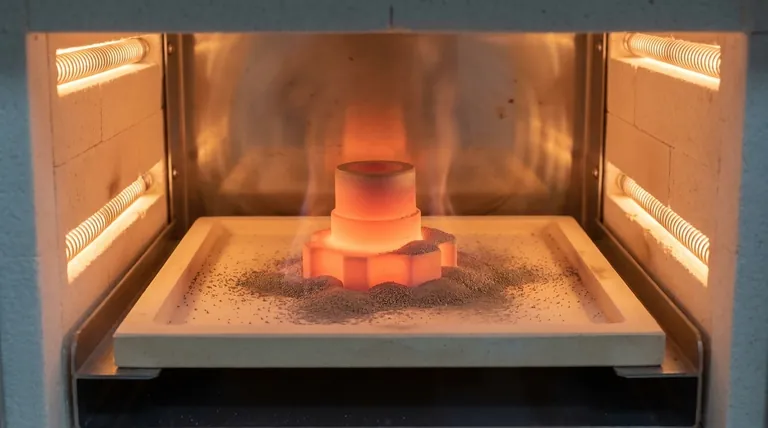
Why Temperature Is the Key to Sentry
Sintering is fundamentally a process of atomic transportation driven by heat. Understanding this principle is more important than memorizing a specific temperature, as it allows you to adapt the process to different materials and goals.
Activating Atomic Diffusion
Heat gives the atoms within the metal powder particles kinetic energy. At the sintering temperature, atoms become mobile enough to move from one particle to another at their points of contact.
This movement, known as atomic diffusion, fills the voids (porosity) between the particles, effectively "welding" them together on a microscopic level.
A Solid-State Process
Crucially, sintering happens below the material's melting point. If the metal were to melt, the precisely formed shape of the pre-sintered component (the "green part") would be lost.
The goal is to fuse the particles, not liquefy them. This is why the process is often called solid-state sintering.
The "0.6 Tm" Rule of Thumb
The guideline of using a temperature greater than 0.6 times the melting point (Tm) provides a reliable starting point. For example, iron melts at 1538°C (1811 K).
A typical sintering temperature for iron-based powders would therefore be above approximately 923°C (0.6 * 1538°C), often in the range of 1120°C to 1150°C to achieve optimal properties.
Factors That Influence the Ideal Temperature
The "0.6 Tm" rule is a starting point, not a final answer. The ideal temperature for your specific application is influenced by several interconnected factors.
The Specific Metal or Alloy
This is the most significant factor. A low-melting-point metal like aluminum (melts at 660°C) will sinter at a far lower temperature than a refractory metal like tungsten (melts at 3422°C).
Compaction and Green Density
The density of the component before sintering (its "green density") plays a role. A more highly compacted part has less empty space between particles and may require less aggressive temperature profiles to achieve full density.
Particle Size
Finer metal powders have a much higher surface-area-to-volume ratio. This provides more pathways for diffusion and can sometimes allow for successful sintering at slightly lower temperatures or for shorter times.
Sintering Atmosphere
Most metals will readily oxidize at high temperatures. Oxidation on the particle surfaces will prevent them from fusing together.
Therefore, sintering must be done in a controlled atmosphere, such as a vacuum or under a protective shielding gas (like nitrogen, argon, or endothermic gas), to prevent oxidation and ensure proper diffusion.
Understanding the Trade-offs
Selecting a sintering temperature is an exercise in balancing competing objectives. There is no single "best" temperature, only the right one for a specific outcome.
Temperature vs. Time
There is an inverse relationship between sintering temperature and time. You can often achieve a similar level of density by sintering at a lower temperature for a longer time, or at a higher temperature for a shorter time.
The Risk of Grain Growth
Using an excessively high temperature or holding the part at temperature for too long can cause grain growth. This is where the individual crystalline grains within the metal merge and grow larger.
Excessive grain growth can degrade the mechanical properties of the final part, particularly its strength and toughness.
Distortion and Dimensional Accuracy
The higher the temperature, the greater the risk of slumping, warping, or other forms of thermal distortion. For components requiring high dimensional precision, a carefully controlled, often lower, temperature is critical.
Making the Right Choice for Your Goal
Your ideal sintering temperature is dictated by your end goal. Use these principles to guide your process development.
- If your primary focus is achieving maximum density and strength: You will likely operate in the upper range of the recommended temperature for that alloy to maximize diffusion, while carefully managing time to prevent excessive grain growth.
- If your primary focus is maintaining precise dimensional control: A more conservative approach using a lower temperature for a longer duration is often preferred to minimize the risk of distortion.
- If your primary focus is throughput and efficiency: You must find the optimal balance between a higher temperature (which enables faster cycles) and the increased energy costs and process risks involved.
Ultimately, mastering the sintering temperature is about controlling atomic movement to build your component from the ground up.
Summary Table:
| Factor | Influence on Sintering Temperature |
|---|---|
| Metal/Alloy Melting Point (Tm) | Primary determinant; temperature is typically >60% of Tm. |
| Particle Size | Finer powders may allow for slightly lower temperatures. |
| Sintering Atmosphere | Prevents oxidation; does not directly set temperature but is critical for success. |
| Target Density vs. Dimensional Control | Higher density goals require higher temperatures; precision parts need lower temperatures. |
Achieve optimal sintering results for your metal components. Selecting the right temperature is critical for density, strength, and dimensional accuracy. KINTEK specializes in lab equipment and consumables, providing the precise furnace technology and expert support your laboratory needs to master the sintering process. Contact our experts today to discuss your specific metal sintering requirements and discover the right solution for you.
Visual Guide

Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What are the most commonly used metals in a vacuum furnace's hot zone? Discover the Key to High-Purity Processing
- What are the advantages of vacuum firing? Achieve Ultimate Material Purity and Performance
- Is heat Cannot travel in a vacuum True or false? Discover How Heat Crosses the Void of Space
- What happens to heat generated in a vacuum? Mastering Thermal Control for Superior Materials
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service



















