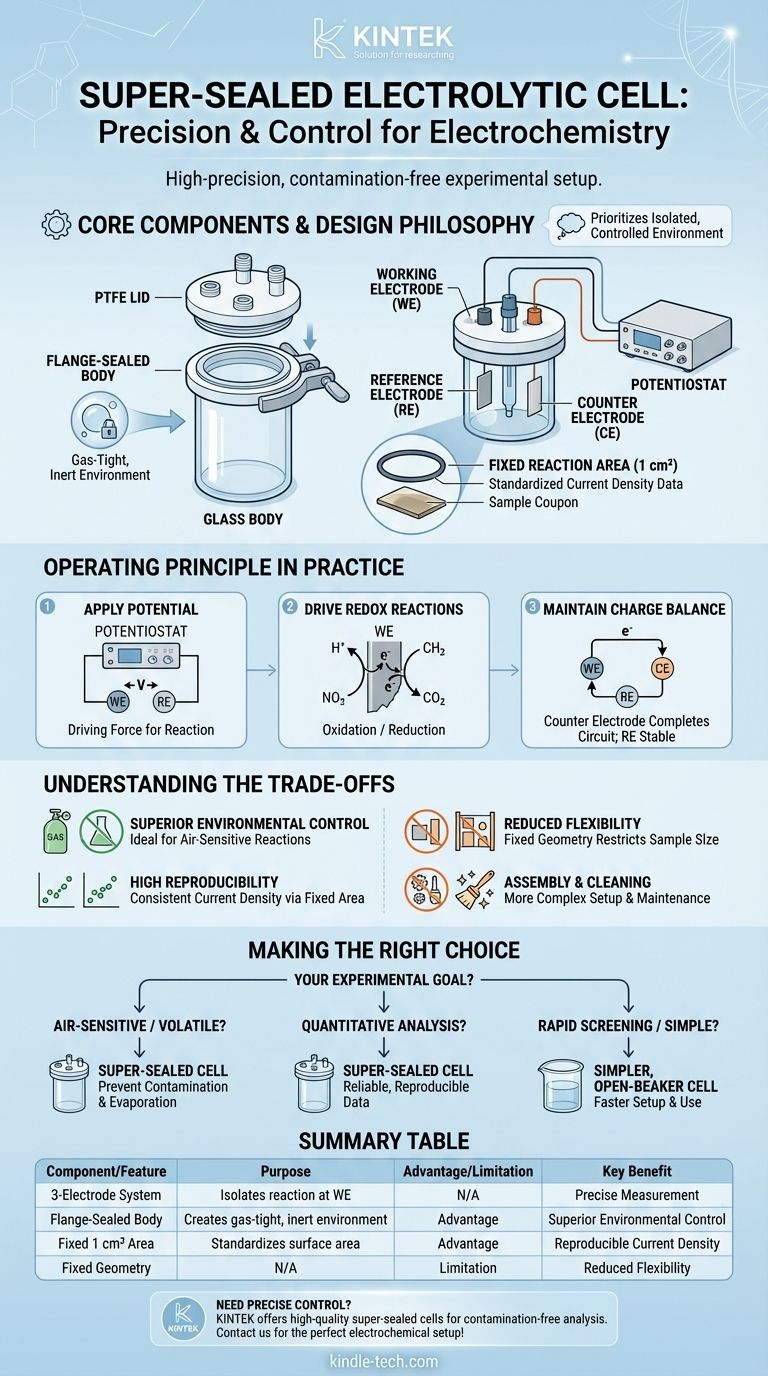
At its core, a super-sealed electrolytic cell is a specialized three-electrode system designed for high-precision electrochemical experiments. It typically consists of a glass body and a Polytetrafluoroethylene (PTFE) lid, which are clamped together with a flange to create a superior, gas-tight seal. Its operating principle is based on applying a controlled voltage to drive redox reactions at a well-defined electrode surface area, enabling highly reproducible and contamination-free measurements.
The defining feature of a super-sealed cell is not just its function, but its design philosophy. It prioritizes a perfectly controlled and isolated environment, making it the standard for experiments where atmospheric contamination or electrolyte evaporation would compromise the results.

Understanding the Core Components
To grasp the cell's function, it's essential to understand the purpose of each component. The design is a direct response to the need for precision and control in modern electrochemistry.
The Three-Electrode System
This is the standard configuration for most electrochemical analysis. It includes a working electrode (WE) where the reaction of interest occurs, a reference electrode (RE) that provides a stable potential to measure against, and a counter electrode (CE) that completes the circuit. This setup isolates the reaction at the WE for precise measurement.
The Flange-Sealed Body
The cell's body is typically made of glass, providing visibility and general chemical resistance. The key innovation is the flange seal. A PTFE cover is pressed firmly against the glass body's flange, creating a much more robust and airtight seal than simple stoppers or ground glass joints.
The use of PTFE (Teflon) for the cover is deliberate. It is extremely chemically inert, preventing contamination or reaction with aggressive electrolytes or solvents.
The Fixed Reaction Area
A critical feature is the defined opening at the bottom of the cell, often a circle with a fixed area of 1 square centimeter. An O-ring is used to seal the sample (the working electrode) against this opening.
This standardization is crucial because it allows for the direct calculation of current density (Amperes per cm²), a fundamental parameter that normalizes results and makes them comparable across different experiments and laboratories.
The Operating Principle in Practice
The cell functions by controlling the electrical potential to drive chemical change. The process is managed by an external instrument called a potentiostat.
Step 1: Applying Potential
The potentiostat applies a precise voltage difference between the working electrode and the reference electrode. This potential acts as the driving force for the electrochemical reaction.
Step 2: Driving Redox Reactions
This applied potential forces either an oxidation (loss of electrons) or a reduction (gain of electrons) to occur at the surface of the working electrode. This is the core chemical event you are studying.
Step 3: Maintaining Charge Balance
As the reaction proceeds at the working electrode, the counter electrode passes an equal and opposite current to keep the overall charge in the cell balanced. This ensures the reference electrode remains stable and is not subjected to high currents, which would compromise its stable potential.
Understanding the Trade-offs
No single piece of equipment is perfect for every task. The super-sealed cell offers distinct advantages but also has practical limitations.
Advantage: Superior Environmental Control
The robust flange seal is the primary benefit. It allows you to purge the cell with an inert gas (like nitrogen or argon) and maintain that atmosphere, which is essential for studying air-sensitive reactions that would be ruined by exposure to oxygen or moisture.
Advantage: High Reproducibility
The fixed, 1 cm² reaction area eliminates a major source of experimental variability. By ensuring the current is always measured over the same surface area, you achieve highly consistent and reproducible current density data.
Limitation: Reduced Flexibility
The fixed geometry can be a drawback. If you need to test working electrodes of various sizes or irregular shapes, this cell design is restrictive unless you acquire custom-made versions.
Limitation: Assembly and Cleaning
Compared to a simple open beaker cell, a flange system is more complex to assemble, disassemble, and clean. This adds overhead to each experiment, making it less ideal for rapid screening of many different samples.
Making the Right Choice for Your Goal
The decision to use a super-sealed cell should be based on your specific experimental needs.
- If your primary focus is air-sensitive electrochemistry or volatile electrolytes: This cell is the ideal choice, as its superior seal prevents atmospheric contamination and sample evaporation.
- If your primary focus is quantitative analysis and comparing results: The standardized reaction area is a critical feature for generating the reliable, reproducible current density data you need.
- If your primary focus is rapid screening or simple material characterization: A simpler, open-beaker cell may be a more efficient choice due to its ease of use and faster setup time.
Choosing a super-sealed cell is a deliberate decision to prioritize environmental control and analytical precision in your electrochemical experiments.
Summary Table:
| Component/Feature | Purpose |
|---|---|
| Three-Electrode System | Isolates reaction at working electrode for precise measurement. |
| Flange-Sealed Body (Glass/PTFE) | Creates a gas-tight, inert environment to prevent contamination. |
| Fixed 1 cm² Reaction Area | Standardizes surface area for reproducible current density data. |
| Potentiostat Control | Applies precise voltage to drive redox reactions. |
| Primary Advantage | Superior environmental control for air-sensitive studies. |
| Key Limitation | Reduced flexibility for testing electrodes of various sizes. |
Need precise control and reproducibility for your electrochemical experiments? KINTEK specializes in high-quality lab equipment, including super-sealed electrolytic cells designed for contamination-free, quantitative analysis. Our solutions ensure you get reliable data for air-sensitive reactions and volatile electrolytes. Contact our experts today to find the perfect electrochemical setup for your laboratory's needs!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- Why is a three-electrode electrolytic cell system used for testing HER? Ensure Precise Catalytic Analysis
- What are the core functions of the electrolytic cell in graphite exfoliation? Engineered Graphene Production
- What role does an electrochemical workstation play in TiNO coating evaluation? Quantify Biological Corrosion Protection
- Why are diaphragm-type electrolytic cells preferred in iron electrowinning? Boost Efficiency and Prevent Re-dissolution
- Why is a single-chamber electrolytic cell equipped with a cooling jacket used? Optimize (Non-)Kolbe Electrolysis
- Why are cooling systems essential for industrial-scale electrolysis cells? Manage Waste Heat for Peak Performance
- What are the necessary preparation steps before using a thin-layer spectroelectrochemical cell? A Guide to Reliable Results
- What is the necessity of a non-woven fabric layer in a manganese electrolytic cell? Ensure High-Purity Metal Production



















