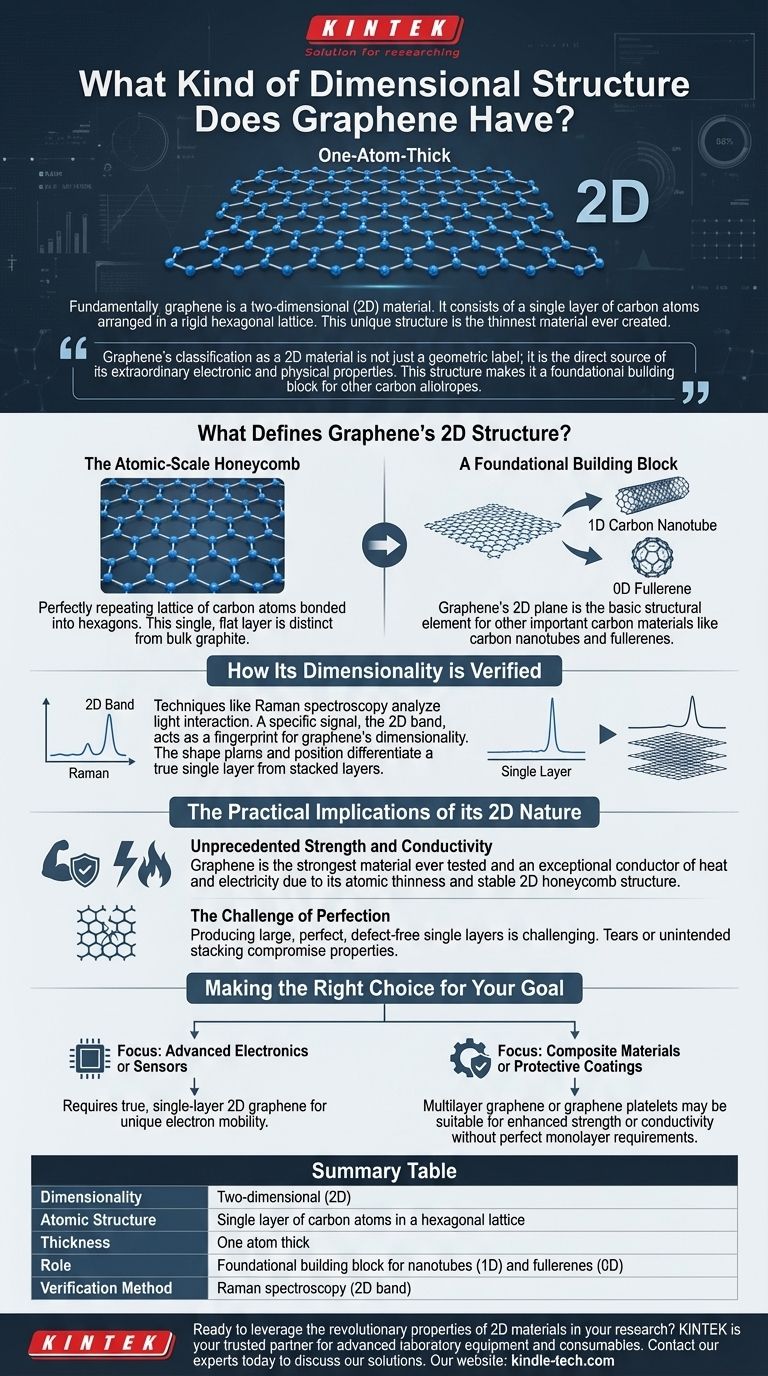
Fundamentally, graphene is a two-dimensional (2D) material. It consists of a single layer of carbon atoms arranged in a rigid hexagonal lattice. This unique, one-atom-thick structure is often described as a honeycomb sheet and is the thinnest material ever created.
Graphene's classification as a 2D material is not just a geometric label; it is the direct source of its extraordinary electronic and physical properties. This structure makes it a foundational building block for other carbon allotropes.

What Defines Graphene's 2D Structure?
The concept of a two-dimensional material is best understood by examining graphene at the atomic level. Its structure is defined by its length and width, with a thickness that is negligible in comparison.
The Atomic-Scale Honeycomb
Graphene's structure is a perfectly repeating lattice of carbon atoms bonded into hexagons, much like a honeycomb. This hexagonal lattice is incredibly strong and stable.
Crucially, this is a single, flat layer. There is no "third dimension" of atoms stacked on top of each other, which is what distinguishes it from its bulk parent material, graphite.
A Foundational Building Block
Graphene's 2D plane is the basic structural element for other important carbon materials. This two-dimensional sheet can be conceptually manipulated to form other allotropes.
If you roll it into a cylinder, you create a one-dimensional (1D) carbon nanotube. If you wrap it into a sphere, you create a zero-dimensional (0D) fullerene.
How Its Dimensionality is Verified
The 2D nature of graphene is not just theoretical; it is a measurable physical property that scientists can verify with precision.
Spectroscopic Fingerprints
Techniques like Raman spectroscopy can analyze how light interacts with the material's atomic structure. A specific signal, known as the 2D band, acts as a fingerprint for graphene's dimensionality.
The shape and position of this 2D band can definitively differentiate between a true single layer and a sample with two, three, or more layers stacked together.
The Importance of a Single Layer
As soon as a second layer is added, the electronic properties begin to change. When many layers are stacked, the material's behavior shifts from the exotic 2D properties of graphene to the more conventional 3D properties of graphite.
The Practical Implications of its 2D Nature
The isolation of this 2D material was so scientifically significant it was awarded the Nobel Prize in Physics in 2010. The reason is simple: confining electrons to a two-dimensional plane unlocks remarkable properties.
Unprecedented Strength and Conductivity
Because of its 2D honeycomb structure, graphene is the strongest material ever tested and an exceptional conductor of heat and electricity. These properties are a direct result of its atomic thinness and stable lattice.
The Challenge of Perfection
The primary challenge with graphene is producing large, perfect, defect-free single layers. Any tear, defect, or unintended stacking can compromise the properties that make it so valuable.
Maintaining its ideal 2D structure during manufacturing and integration into other devices remains a significant area of research and engineering.
Making the Right Choice for Your Goal
Understanding the role of graphene's dimensionality is critical for its application. The term "graphene" is often used loosely, but the number of layers fundamentally dictates performance.
- If your primary focus is advanced electronics or sensors: You require true, single-layer 2D graphene to leverage its unique electron mobility and sensitivity.
- If your primary focus is composite materials or protective coatings: Multilayer graphene or graphene platelets may be perfectly suitable, providing enhanced strength or conductivity without the stringent requirements of a perfect monolayer.
Recognizing that graphene's power comes from its two-dimensional foundation is the key to unlocking its revolutionary potential.
Summary Table:
| Key Aspect | Description |
|---|---|
| Dimensionality | Two-dimensional (2D) |
| Atomic Structure | Single layer of carbon atoms in a hexagonal (honeycomb) lattice |
| Thickness | One atom thick |
| Role | Foundational building block for carbon nanotubes (1D) and fullerenes (0D) |
| Verification Method | Raman spectroscopy (2D band) |
Ready to leverage the revolutionary properties of 2D materials in your research?
KINTEK is your trusted partner for advanced laboratory equipment and consumables. Whether you are synthesizing, characterizing, or integrating graphene and other nanomaterials, our precision tools are designed to meet the stringent demands of cutting-edge science.
Contact our experts today to discuss how our solutions can help you achieve flawless results in your material science projects.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Custom-Made Alumina Zirconia Special-Shaped Ceramic Plates for Engineering Advanced Fine Ceramics Processing
People Also Ask
- What are the advantages of thin film resistors? Precision, Stability & Low Noise for Sensitive Circuits
- What information does a thermocouple at the substrate bottom provide in LCVD? Master Precise Temperature Control
- What is thin film deposition in IC fabrication? Build the Core Layers of Your Microchip
- How does a tube CVD reactor facilitate N-CNT growth? Master Precision Synthesis on Carbon Paper
- What role do porous substrates play in CDCVD beyond acting as a support? Master the Engine of Membrane Growth
- Why is a cooling stage with precise temperature control necessary for iCVD? Master Surface Engineering Thermodynamics
- What are the disadvantages of chemical vapor deposition? High Costs, Safety Risks, and Material Limitations
- What is the advantage of sputtering based thin film deposition? Superior Adhesion & Versatility for High-Quality Films



















