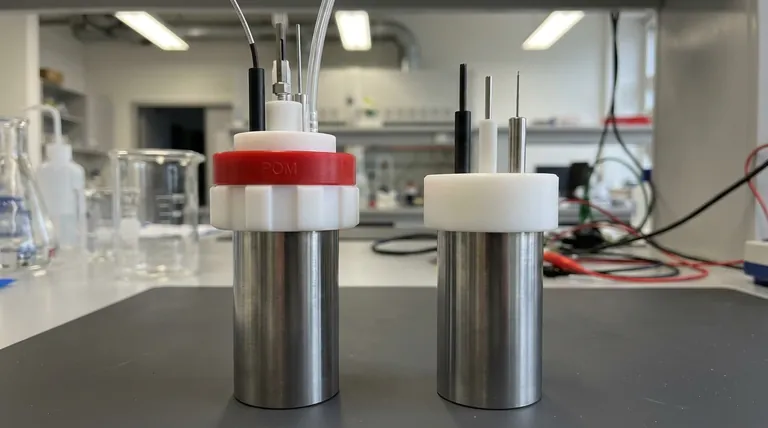
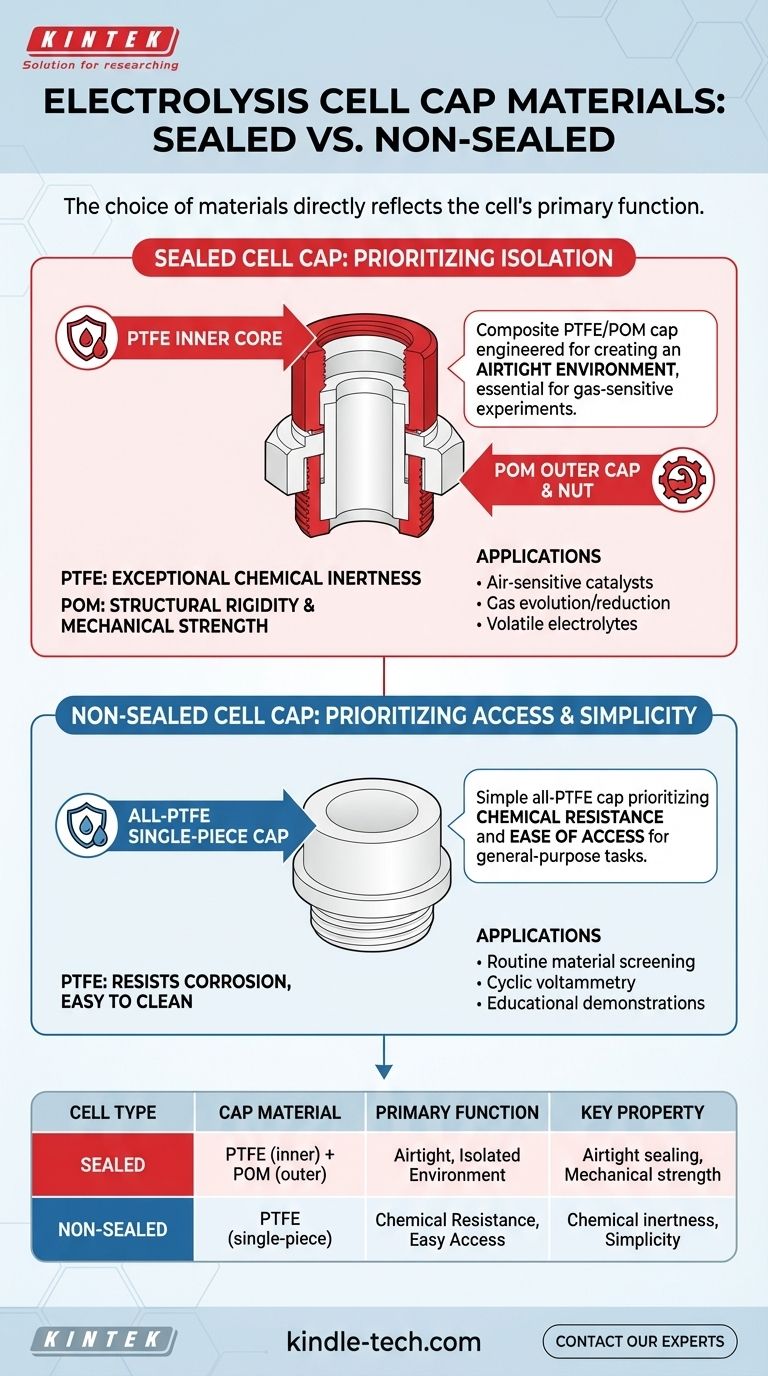
To answer directly, the sealed electrolysis cell uses a multi-part cap made from a Polytetrafluoroethylene (PTFE) inner core, a Polyoxymethylene (POM) outer cap, and a POM screw nut. The non-sealed electrolysis cell, in contrast, uses a simpler, single-piece cap made entirely of Polytetrafluoroethylene (PTFE).
The choice of materials is not arbitrary; it directly reflects the cell's primary function. The sealed cell's composite PTFE/POM cap is engineered for creating an airtight environment, while the non-sealed cell's all-PTFE cap prioritizes chemical resistance and ease of access.
Deconstructing the Cap Materials
To understand why these specific plastics are chosen, we must look at their individual properties and how they work together.
The Role of Polytetrafluoroethylene (PTFE)
PTFE, commonly known by the brand name Teflon, is the material that directly faces the chemical environment inside the cell. It is used for the entire cap in the non-sealed version and for the inner core of the sealed version.
This choice is due to PTFE's exceptional chemical inertness. It resists corrosion from nearly all acids, bases, and solvents used in electrochemistry, ensuring the cell does not contaminate the experiment or degrade over time.
The Function of Polyoxymethylene (POM)
POM, also known as acetal or Delrin, is a high-strength engineering plastic used for the outer components of the sealed cell cap—the red cap and the white screw nut.
Unlike the chemically resistant but relatively soft PTFE, POM provides structural rigidity and mechanical strength. This is critical for the "external thread sealing structure," as the POM components can be tightened to apply consistent pressure and create an airtight seal without deforming.
The Composite Design of the Sealed Cap
The sealed cell cap is a perfect example of purpose-driven engineering. The design combines the strengths of two different materials.
The PTFE inner core handles the chemical exposure, protecting the experiment. The outer POM cap and nut provide the mechanical force needed to secure the lid and maintain a controlled, isolated atmosphere within the cell.
Why the Designs Differ: Sealed vs. Non-Sealed Functionality
The material and structural differences between the two caps directly correspond to the types of experiments each cell is designed for.
The Sealed Cell: Prioritizing Isolation
A sealed electrolysis cell is necessary for experiments that are sensitive to the ambient atmosphere. This includes studying reactions involving gases (like oxygen reduction or CO₂ conversion) or working with volatile electrolytes.
The threaded POM/PTFE cap is essential for creating the airtight seal required for these tasks. The multiple openings (five in the standard model) allow for the insertion of electrodes, gas lines for purging, and sampling ports, all while maintaining the integrity of the sealed environment.
The Non-Sealed Cell: Prioritizing Access and Simplicity
A non-sealed cell is the workhorse for general-purpose electrochemistry where atmospheric contact is not a critical variable. This includes routine material screening, cyclic voltammetry, and educational demonstrations.
The single-piece PTFE cap is ideal for this role. It prevents splashes and reduces evaporation while allowing for quick and easy access to the cell's interior. The fewer, uniform openings are sufficient for holding the standard three-electrode setup.
Understanding the Trade-offs
Choosing between these cells involves balancing experimental needs against complexity and cost.
Sealed Cell: Precision vs. Complexity
The primary advantage of the sealed cell is its ability to create a controlled, isolated environment, which is non-negotiable for certain research.
The trade-off is increased complexity. The multi-part cap requires more care during assembly and disassembly. This design is typically more expensive due to the more intricate manufacturing and additional components.
Non-Sealed Cell: Simplicity vs. Exposure
The non-sealed cell's key benefit is its simplicity. It is robust, easy to clean, and straightforward to set up, making it highly efficient for high-throughput testing.
Its limitation is the lack of atmospheric control. It is unsuitable for experiments sensitive to oxygen or carbon dioxide, and evaporation can alter the electrolyte concentration during long-duration experiments.
Making the Right Choice for Your Experiment
Your experimental goal should be the sole driver of your choice between a sealed and non-sealed cell.
- If your primary focus is on air-sensitive catalysts, gas evolution/reduction studies, or volatile electrolytes: The sealed cell is essential, as its POM/PTFE sealing mechanism is specifically designed for atmospheric isolation.
- If your primary focus is on routine material screening, educational demonstrations, or general cyclic voltammetry: The non-sealed cell's simple all-PTFE cap offers the best balance of chemical resistance and ease of use.
- If your primary focus is on building custom setups: Note that both cell types may allow for customized openings, but the sealed cell's default configuration with multiple port sizes offers greater initial flexibility for complex arrangements.
By understanding how material selection serves the cell's core function, you can confidently choose the precise tool required for reliable and accurate results.
Summary Table:
| Cell Type | Cap Material(s) | Primary Function | Key Property |
|---|---|---|---|
| Sealed | PTFE (inner core), POM (outer cap & nut) | Create an airtight, isolated environment | Airtight sealing, mechanical strength |
| Non-Sealed | PTFE (single-piece) | Provide chemical resistance and easy access | Chemical inertness, simplicity |
Ready to choose the right electrolysis cell for your experiment?
Whether you need the airtight precision of a sealed cell or the robust simplicity of a non-sealed design, KINTEK has the solution. Our specialized lab equipment ensures reliable performance for your electrochemistry research, from gas evolution studies to routine material screening.
Contact our experts today to discuss your specific needs and let KINTEK provide the reliable equipment you need for accurate results.
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are the standard opening specifications for all-PTFE electrolytic cells? A Guide to Sealed vs. Non-Sealed Ports
- What are the key material properties and structural features of an all-PTFE electrolytic cell? Achieve Unmatched Purity in Harsh Electrochemical Environments
- What is the precaution regarding temperature when using an all-PTFE electrolytic cell? Essential Thermal Safety Tips
- What are the typical volumes for an all-PTFE electrolytic cell? Choose the Right Size for Your Experiment
- How should an all-PTFE electrolytic cell be handled to prevent mechanical damage? Protect Your Investment and Data Integrity



















