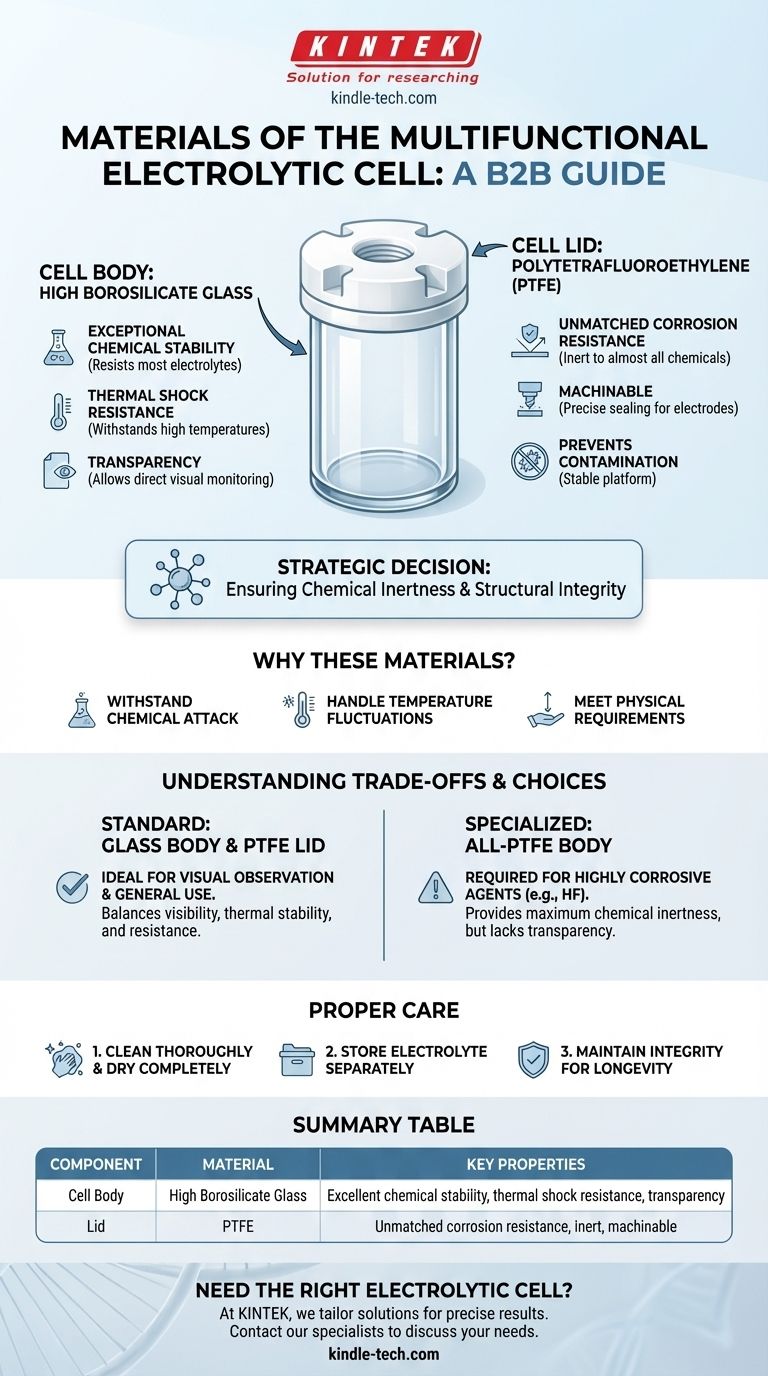
To be precise, the body of a standard multifunctional electrolytic cell is constructed from high borosilicate glass, while the lid is precision-machined from Polytetrafluoroethylene (PTFE). This combination is deliberately chosen to ensure the chemical inertness and structural integrity required for accurate electrochemical analysis.
The selection of high borosilicate glass and PTFE is a strategic engineering decision. These materials provide the necessary chemical resistance and thermal stability to contain highly reactive electrolytes without contaminating the experiment or degrading over time.

Why These Specific Materials Are Chosen
The function of an electrolytic cell—to drive non-spontaneous chemical reactions—creates a demanding internal environment. The materials used for its construction must withstand chemical attack, temperature fluctuations, and the physical requirements of the setup.
The Role of the Cell Body: High Borosilicate Glass
The primary function of the cell body is to safely contain the electrolyte and the submerged electrodes.
High borosilicate glass is the material of choice for this component due to its exceptional properties. It offers excellent chemical stability, meaning it will not react with or leach impurities into the vast majority of electrolyte solutions.
Furthermore, its resistance to high temperatures and thermal shock is critical for experiments that generate heat. Its transparency is also a key practical advantage, allowing for direct visual monitoring of the reaction.
The Function of the Lid: Polytetrafluoroethylene (PTFE)
The lid serves to seal the cell, prevent contamination from the outside environment, and provide a stable platform for holding the electrodes in place.
PTFE (often known by the brand name Teflon) is used for its virtually unmatched corrosion resistance. It is inert to almost all chemicals, which is essential as it is in close proximity to the electrolyte and any fumes produced.
This material is also easily machined, allowing for the creation of precise, tightly-sealed openings for the working electrode, counter electrode, and reference electrode that make up the common three-electrode system.
Understanding the Trade-offs
While the glass-and-PTFE combination is the most common, it is not the only option. The optimal choice is always dictated by the specific demands of the experiment.
Glass vs. PTFE for the Cell Body
For most applications, a high borosilicate glass body is ideal due to its clarity and thermal properties.
However, in specialized cases involving substances that can etch glass (such as hydrofluoric acid), a cell body made entirely of PTFE may be required. This provides the ultimate chemical resistance, but the clear trade-off is the complete loss of transparency.
The Importance of Proper Care
Even these highly resistant materials require proper handling to ensure longevity and prevent experimental contamination.
After use, the cell should be thoroughly cleaned and completely dried. For long-term storage, it is critical to pour out the electrolyte and store it in a separate, sealed container to maintain the integrity of both the cell and the solution.
Making the Right Choice for Your Goal
The material selection for an electrolytic cell is fundamental to the quality of your results. Your decision should be based on the specific chemical system you are investigating.
- If your primary focus is visual observation and general-purpose use: A high borosilicate glass body with a PTFE lid is the industry standard for its balance of visibility, thermal stability, and chemical resistance.
- If your primary focus is working with highly corrosive agents that attack glass: An all-PTFE cell body is the superior choice, providing maximum chemical inertness at the cost of transparency.
- If your primary focus is maintaining absolute experimental purity: Both materials are excellent, but you must implement a strict cleaning and drying protocol between experiments to prevent cross-contamination.
Ultimately, understanding the properties of your equipment's materials is the first step toward achieving reliable and repeatable electrochemical data.
Summary Table:
| Component | Material | Key Properties |
|---|---|---|
| Cell Body | High Borosilicate Glass | Excellent chemical stability, thermal shock resistance, transparency for visual monitoring |
| Lid | PTFE (Polytetrafluoroethylene) | Unmatched corrosion resistance, inert to chemicals, easily machined for precise electrode sealing |
Need the Right Electrolytic Cell for Your Specific Application?
At KINTEK, we specialize in providing high-quality lab equipment, including electrolytic cells tailored to your experimental needs. Whether you require the standard high borosilicate glass body for visual observation or a fully PTFE construction for handling highly corrosive agents, our experts can help you select the perfect solution for accurate and reliable electrochemical data.
Let us help you achieve precise results: Contact our lab equipment specialists today to discuss your requirements and find the ideal electrolytic cell for your laboratory.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What is the function of Alumina Sheaths in a molten salt electrolytic cell assembly? Essential High-Heat Protection
- How does current density influence PEO ceramic coatings? Master Precision Power for Superior Surface Quality
- What is the purpose of utilizing industrial-grade electrolytic cells and circulation pumps? Expert Scale-Up Guide
- What is the primary benefit of micro-electrochemical cells? Maximize Research with Minimal Reagents
- What are the required steps before using a super-sealed electrolytic cell? Ensure Safety and Data Integrity
- What role do laboratory-grade cylindrical borosilicate glass reactors play? Enhance Your Electro-Oxidation Research
- What is the correct shutdown procedure after an experiment? A Step-by-Step Guide to Safe Deactivation
- How do electrode spacing and monopolar parallel connections affect electrolytic performance? Optimize Energy & Efficiency



















