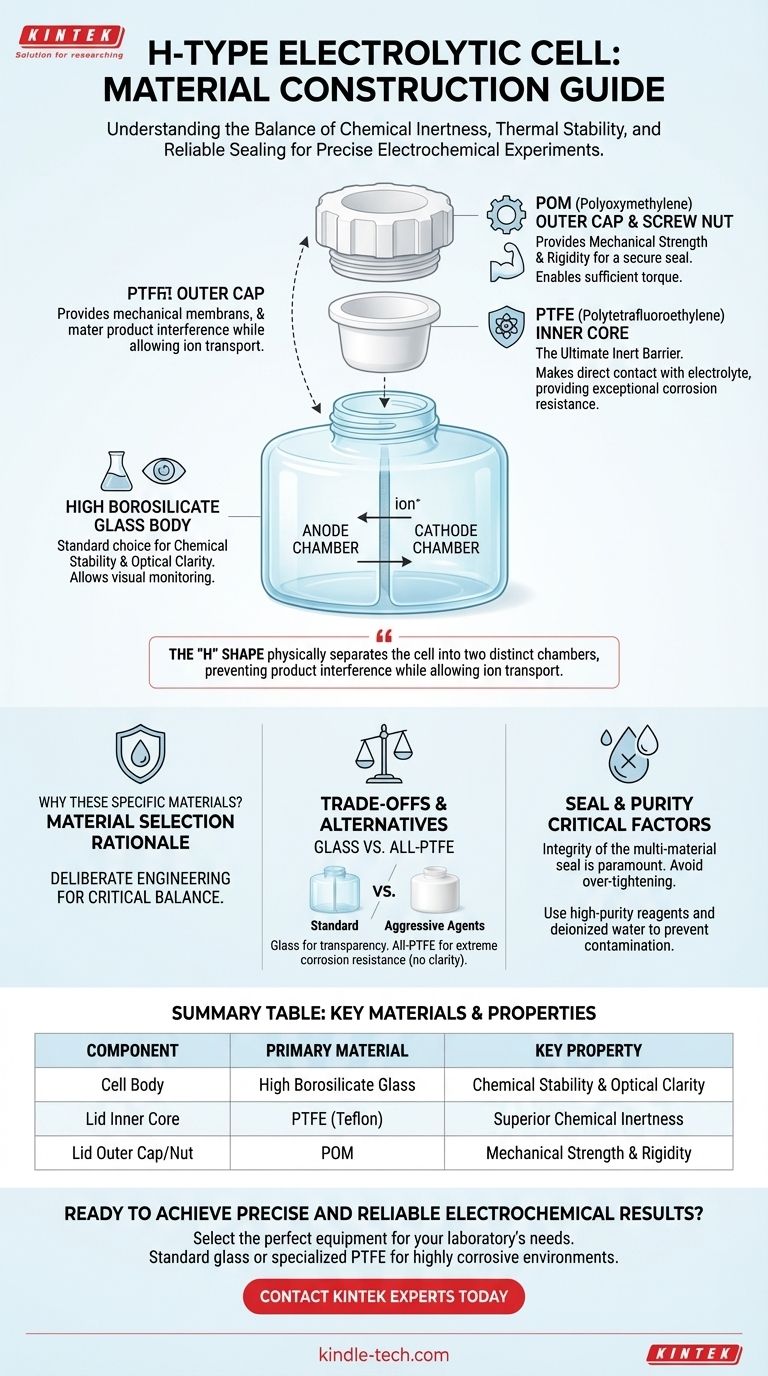
At its core, an H-type electrolytic cell body is constructed from high borosilicate glass. The lid is a more complex, multi-part assembly featuring a PTFE (Polytetrafluoroethylene) inner core that makes direct contact with the internal environment, and an outer sealing cap made of POM (Polyoxymethylene) for mechanical strength.
The selection of these materials is not arbitrary; it is a deliberate engineering choice to achieve a critical balance between chemical inertness, thermal stability, and a reliable seal, which are all essential for conducting accurate and repeatable electrochemical experiments.

The Anatomy of an H-Type Cell
The "H" shape of the cell is fundamental to its function. It physically separates the cell into two distinct chambers, one for the anode and one for the cathode.
The Glass Body
The main body of the cell is almost universally made from high borosilicate glass. This structure allows for the use of a replaceable ion exchange membrane between the two chambers.
This separation ensures that the reactions at each electrode can proceed independently without the products interfering with one another, while still allowing for necessary ion transport.
The Multi-Component Lid
The lid is engineered to provide an effective seal. It uses an external thread design composed of several materials working in concert.
The inner core of the lid is made from PTFE. This is the component that faces the electrolyte and any vapors, providing a chemically inert barrier.
The outer cap and screw nut are made from POM. These parts provide the rigidity and mechanical force needed to tighten the lid and create a secure seal around the cell's openings.
Why These Specific Materials Are Chosen
Each material serves a distinct purpose, leveraging its unique properties to contribute to the cell's overall performance and reliability.
High Borosilicate Glass: For Clarity and Stability
This type of glass is the standard for high-performance lab equipment. Its primary benefits are excellent chemical stability and resistance to a wide range of substances.
It also offers high-temperature resistance and, crucially, optical transparency, allowing researchers to visually monitor the experiment.
PTFE (Teflon): The Ultimate Inert Barrier
PTFE is renowned for its exceptional corrosion resistance. It is inert to almost all chemicals, making it the perfect material to form the primary seal against the electrolyte.
By using PTFE for the inner core of the lid, the cell ensures that no reactive material compromises the experiment or degrades the equipment.
POM (Polyoxymethylene): For Mechanical Strength
While PTFE is chemically resistant, it is a relatively soft material. POM, an engineering plastic, provides the rigidity and structural strength required for the screw mechanism.
This allows the user to apply sufficient torque to the lid, compressing the inner PTFE core to form a tight, reliable seal without the risk of stripping threads or cracking the component.
Understanding the Trade-offs and Alternatives
While the glass/PTFE/POM combination is the most common, understanding its limitations and alternatives is key to proper experimental design.
Glass vs. All-PTFE Cells
For experiments involving extremely corrosive agents that can etch glass (such as hydrofluoric acid), an alternative cell body made entirely of PTFE may be required.
The trade-off here is clear: you gain superior chemical resistance at the cost of the optical transparency that a glass body provides.
The Importance of the Seal
The effectiveness of the entire system relies on the integrity of the multi-material seal. Over-tightening the POM cap can damage the threads or the glass body.
Likewise, degradation of the PTFE core can lead to leaks, introducing contaminants or allowing the electrolyte to escape, invalidating experimental results.
Purity is Paramount
The choice of chemically inert materials highlights a broader principle in electrochemistry: avoiding contamination. This extends to the chemicals used, which should be prepared with high-purity reagents and deionized water to prevent impurities from interfering with the reactions.
Making the Right Choice for Your Experiment
Your experimental goals should dictate your material considerations.
- If your primary focus is general electrochemistry with standard aqueous or organic electrolytes: The high borosilicate glass body with a PTFE/POM lid is the ideal and most common configuration.
- If you are working with highly aggressive or glass-etching agents: You must use a cell constructed entirely from PTFE to ensure chemical compatibility and prevent equipment failure.
- If maintaining a perfectly sealed, oxygen-free environment is your priority: Meticulously inspect the integrity of the PTFE core and POM screw threads before every experiment, as this seal is your most critical component.
Ultimately, understanding the function of each material empowers you to control your experimental variables with precision and confidence.
Summary Table:
| Component | Primary Material | Key Property |
|---|---|---|
| Cell Body | High Borosilicate Glass | Chemical Stability & Optical Clarity |
| Lid Inner Core | PTFE (Teflon) | Superior Chemical Inertness |
| Lid Outer Cap/Nut | POM | Mechanical Strength & Rigidity |
Ready to achieve precise and reliable electrochemical results?
The materials of your H-type cell are critical for experimental success. At KINTEK, we specialize in high-quality lab equipment, including electrolytic cells constructed from the optimal materials for your specific application—whether it's standard high borosilicate glass or specialized PTFE for highly corrosive environments.
Let us help you select the perfect equipment for your laboratory's needs. Contact our experts today to discuss your requirements and ensure your experiments are built on a foundation of quality and reliability.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments



















