In practice, no metal is completely immune to induction heating, but the efficiency varies so dramatically that some are considered impractical to heat. Metals that are poor candidates for induction are typically non-magnetic and have very low electrical resistivity. Pure silver, copper, and gold are the most common examples, as they require significantly more power and specialized frequencies to heat effectively compared to materials like iron and steel.
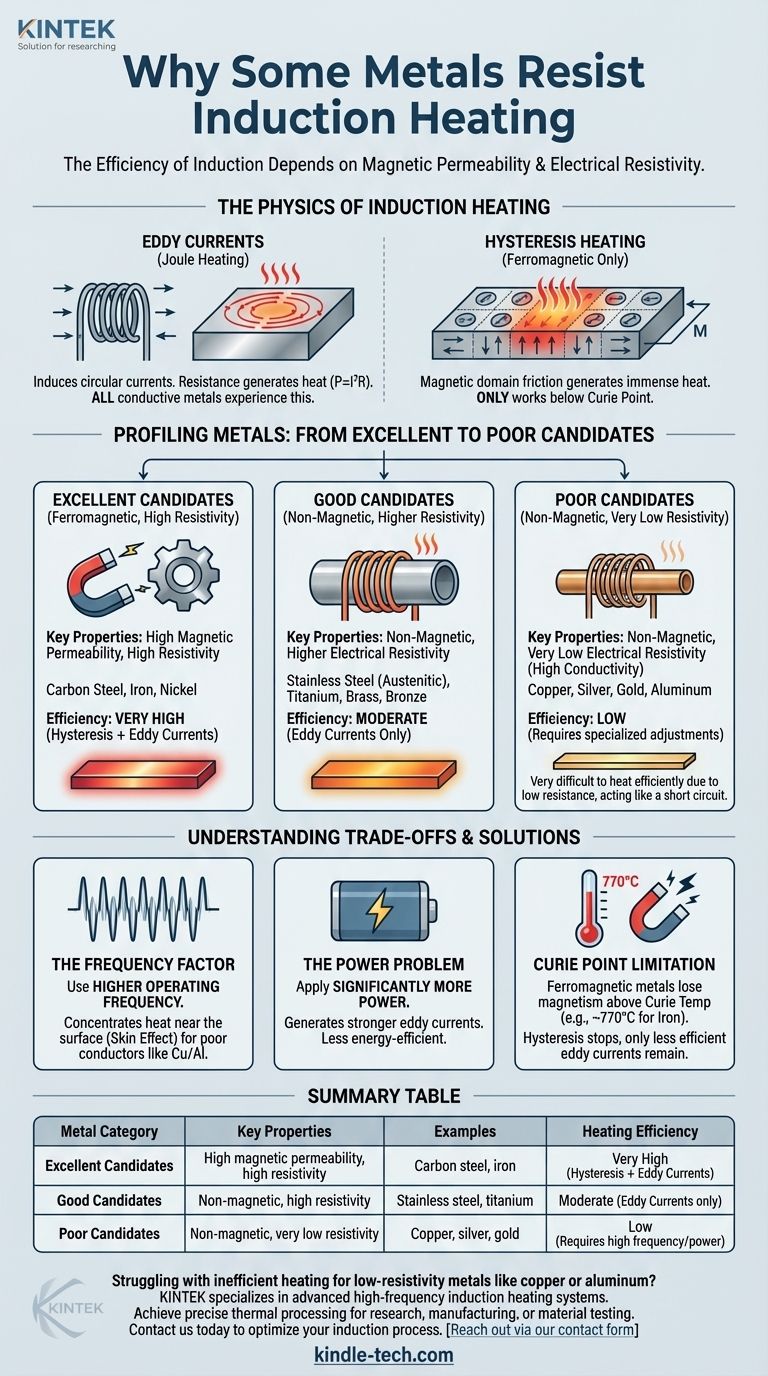
The question isn't whether a metal can be heated by induction, but how efficiently it can be done. A metal's suitability for induction is determined almost entirely by two physical properties: its magnetic permeability and its electrical resistivity.

The Physics of Induction Heating
To understand why some metals are poor candidates, you first need to understand the two mechanisms that generate heat in an induction process.
The Role of Eddy Currents
An induction coil generates a strong, rapidly alternating magnetic field. When a conductive material like a metal is placed inside this field, the field induces circular electrical currents within the metal. These are called eddy currents.
As these currents swirl through the material, they encounter resistance. This resistance to the flow of electricity generates heat, a principle known as Joule heating (P = I²R). Every metal, as a conductor, will experience this effect.
The Power of Hysteresis
For a specific class of metals known as ferromagnetic materials (like iron and certain types of steel), a second, more powerful heating effect occurs.
These materials are composed of tiny magnetic regions called domains. The alternating magnetic field forces these domains to rapidly flip their polarity, aligning back and forth with the field millions of times per second. This internal friction creates immense heat.
Hysteresis heating is extremely efficient, but it only works on magnetic materials and only below a specific temperature known as the Curie point.
Profiling Metals: From Excellent to Poor Candidates
Metals can be grouped into three categories based on how well they respond to induction.
Excellent Candidates: Ferromagnetic Metals
These metals are the easiest and most efficient to heat with induction. They benefit from both eddy currents and hysteresis.
- Examples: Carbon steel, iron, nickel.
- Why they work: They possess both high magnetic permeability (which enables hysteresis heating) and relatively high electrical resistivity (which helps generate more heat from eddy currents).
Good Candidates: Non-Magnetic, Higher Resistivity Metals
These metals are not magnetic, so they only heat via eddy currents. However, their higher electrical resistivity makes this process reasonably efficient.
- Examples: Stainless steel (austenitic grades), titanium, brass, bronze.
- Why they work: While they lack the benefit of hysteresis, their internal resistance is high enough to generate significant heat from the induced eddy currents.
Poor Candidates: Non-Magnetic, Low Resistivity Metals
These are the most challenging metals to heat. They are not magnetic and are such excellent electrical conductors that eddy currents flow with very little resistance, generating minimal heat.
- Examples: Copper, silver, gold, aluminum.
- Why they are difficult: Their very low resistivity (high conductivity) is the primary issue. You can think of it like a short circuit; the current flows easily but doesn't do much "work" in the form of heat.
Understanding the Trade-offs and Solutions
Just because a metal is a "poor" candidate does not make it impossible to heat. The process is simply less efficient and requires specific adjustments.
The Frequency Factor
The key to heating poor conductors like copper or aluminum is to use a much higher operating frequency. Higher frequencies force the eddy currents into a smaller area near the surface of the metal (an effect known as the skin effect), concentrating the heating effect and making the process viable.
The Power Problem
Overcoming low resistivity can also be a matter of brute force. By applying significantly more power to the induction coil, you can generate strong enough eddy currents to heat the material. However, this is far less energy-efficient and can increase operational costs.
The Curie Point Limitation
It is critical to remember that even the best ferromagnetic materials have a limit. Once heated above their Curie temperature (around 770°C or 1420°F for iron), they lose their magnetic properties. Above this point, hysteresis heating stops completely, and the metal heats only through the less efficient eddy current effect.
Making the Right Choice for Your Goal
Your material selection or process design depends entirely on your objective.
- If your primary focus is rapid, efficient heating: Choose a ferromagnetic material like carbon steel or iron whenever possible.
- If you must heat a poor conductor like copper or aluminum: Be prepared to use specialized equipment with higher frequencies and power, and accept lower overall energy efficiency.
- If you need a material to resist induction heating: A highly conductive, non-magnetic material like pure aluminum or silver is a good choice, though a non-metallic material like a ceramic is the only way to ensure no heating occurs.
Ultimately, mastering an induction process comes down to managing the interplay between the material's properties and the frequency of the magnetic field.
Summary Table:
| Metal Category | Key Properties | Examples | Heating Efficiency |
|---|---|---|---|
| Excellent Candidates | High magnetic permeability, high resistivity | Carbon steel, iron | Very High (Hysteresis + Eddy Currents) |
| Good Candidates | Non-magnetic, high resistivity | Stainless steel, titanium | Moderate (Eddy Currents only) |
| Poor Candidates | Non-magnetic, very low resistivity | Copper, silver, gold | Low (Requires high frequency/power) |
Struggling with inefficient heating for low-resistivity metals like copper or aluminum? KINTEK specializes in advanced lab equipment, including high-frequency induction heating systems designed to handle challenging materials. Our expertise ensures you achieve precise thermal processing, whether for research, manufacturing, or material testing. Contact us today to optimize your induction heating process and enhance your lab's efficiency. Reach out via our contact form to discuss your specific needs!
Visual Guide
Related Products
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Lab-Scale Vacuum Induction Melting Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory High Pressure Vacuum Tube Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What are the advantages of using Vacuum Hot Pressing (VHP) equipment? Achieve Superior ODS Steel Density & Structure
- How does the mechanical pressure from a vacuum hot-pressing furnace facilitate the densification of B4C/Al composites?
- Why is the vacuum system of a Vacuum Hot Pressing furnace critical for ODS ferritic stainless steel performance?
- How does high vacuum protect copper composites during sintering? Achieve Pure, Dense Materials with KINTEK Solutions
- How does a vacuum hot press furnace contribute to high-density Cr-50 wt% Si alloys? Achieve Superior Densification



















