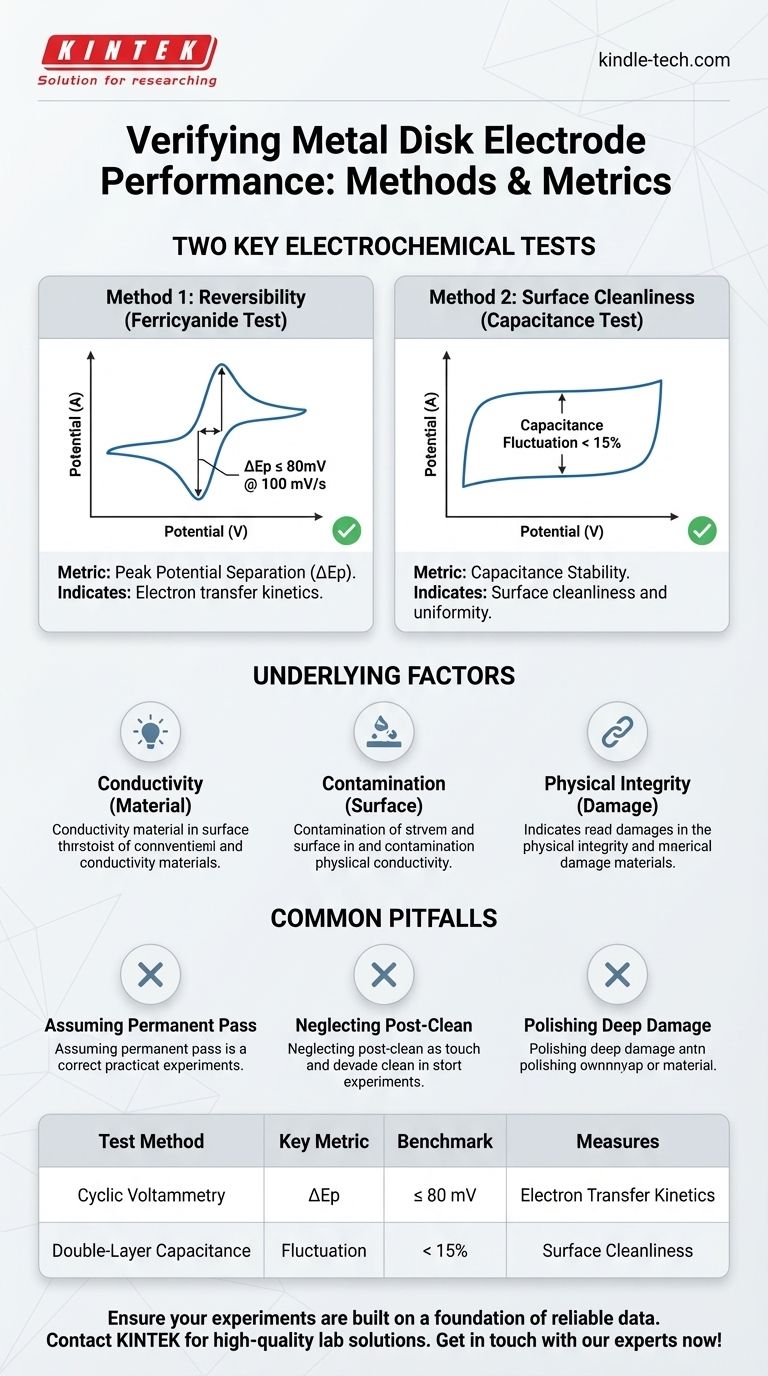
To verify the performance of a metal disk electrode, you should conduct two key electrochemical tests. First, run a cyclic voltammogram in a potassium ferricyanide solution to check its reversibility. Second, measure its double-layer capacitance in a simple salt solution to assess surface cleanliness and uniformity. These quantitative tests provide a clear, data-driven assessment of the electrode's readiness for an experiment.
Verifying an electrode's performance is not a one-time check but a continuous process. True reliability comes from understanding that these tests are proxies for two critical factors: the speed of electron transfer (reversibility) and the cleanliness of the electrode surface.

The Core Performance Metrics
To move beyond a simple visual check, two standard electrochemical tests provide quantitative benchmarks for your electrode's health. They directly measure the properties required for accurate and repeatable results.
Method 1: The Ferricyanide Test for Reversibility
The most common verification is a cyclic voltammetry (CV) experiment using a standard redox couple, typically potassium ferricyanide/ferrocyanide. The key metric you are looking for is the peak potential separation (ΔEp).
This value represents the difference in voltage between the oxidation peak and the reduction peak. For a healthy electrode, this value should be low, indicating fast and efficient electron transfer kinetics.
A standard benchmark is a ΔEp less than or equal to 80mV at a scan rate of 100 mV/s. A value significantly higher than this suggests a sluggish electrode, often due to surface contamination.
Method 2: Double-Layer Capacitance for Surface Cleanliness
This test measures the electrical double-layer capacitance of your electrode in an inert electrolyte solution, such as 0.1M KCl. You are not looking for a specific capacitance value, but rather its stability and fluctuation.
A clean, uniform electrode surface will produce a stable, rectangular-shaped CV curve in a region with no redox reactions. The current measured is purely capacitive.
The performance benchmark here is a fluctuation of less than 15%. High fluctuation or a distorted CV shape points to a non-uniform or contaminated surface, which can interfere with your measurements.
Underlying Factors That Dictate Performance
Passing or failing these tests is a direct result of the electrode's fundamental physical and chemical state. Understanding these factors helps you diagnose and prevent problems.
Electrical Conductivity and Material Choice
An electrode must be highly conductive to function. Materials like gold, platinum, and silver are chosen for their excellent conductivity, which ensures efficient current flow and minimizes signal distortion.
While the base material provides this property, its effective conductivity can be compromised. This is not about the bulk metal failing, but about what happens at the surface.
The Impact of Surface Contamination
Contamination is the primary cause of poor electrode performance. A thin layer of dirt, grease, or oxides on the surface acts as an insulator, increasing resistance.
This added resistance impedes electron transfer, which directly leads to a higher ΔEp in the ferricyanide test and inconsistent results in capacitance measurements.
The Critical Role of Physical Integrity
Before any chemical test, a visual inspection is crucial. Scratches, pits, or physical deformation on the disk surface create non-uniform current densities.
These defects disrupt the electrochemical environment at the electrode face, leading to distorted signals and making your results unreliable and difficult to interpret, even if the electrode is chemically clean.
Common Pitfalls to Avoid
Achieving reliable data requires more than just a good electrode; it demands a good process. Avoiding these common mistakes is essential for maintaining performance over time.
Assuming a "Passing" Grade is Permanent
An electrode that passes verification today can fail tomorrow. Performance is a snapshot in time, heavily dependent on its most recent use and cleaning. Verification is not a one-time setup step but a routine part of your experimental workflow.
Neglecting Post-Experiment Cleaning
The most common cause of performance degradation is skipping immediate and thorough cleaning. Residual electrolyte and reaction byproducts will dry and adsorb onto the surface, creating a stubborn contamination layer.
Always clean the electrode with appropriate solvents (like deionized water or ethanol) immediately after use, dry it, and store it properly in a protected case.
Trying to Polish Away Deep Damage
While polishing can restore a mildly contaminated or passivated surface, it cannot fix deep physical damage. Deep scratches or pits fundamentally alter the electrode's geometry and are often impossible to correct. If an electrode is physically damaged, it must be replaced.
Making the Right Choice for Your Goal
Consistent verification is the foundation of trustworthy electrochemical data. Your approach should align with the sensitivity of your experiment.
- If your primary focus is routine qualitative screening: A quick visual inspection and a ferricyanide test to ensure ΔEp is within the acceptable range are sufficient.
- If your primary focus is high-precision quantitative analysis: You must perform both the ferricyanide and capacitance tests, aiming for a ΔEp well below the 80mV threshold and highly stable capacitance.
- If you are troubleshooting inconsistent results: Methodically check every factor, starting with a visual inspection, followed by a rigorous cleaning protocol, and finally running both verification tests to isolate the problem.
Ultimately, treating your electrode with consistent care and validating its performance before each critical experiment is the only way to guarantee the integrity of your results.
Summary Table:
| Test Method | Key Metric | Performance Benchmark | What It Measures |
|---|---|---|---|
| Cyclic Voltammetry (in Ferricyanide) | Peak Potential Separation (ΔEp) | ≤ 80 mV at 100 mV/s | Electron transfer kinetics and reversibility |
| Double-Layer Capacitance (in KCl) | Capacitance Fluctuation | < 15% | Surface cleanliness and uniformity |
Ensure your experiments are built on a foundation of reliable data. Proper electrode performance is critical for accurate electrochemical analysis. KINTEK specializes in high-quality lab equipment and consumables, including electrodes and accessories, to support your laboratory's needs. Contact us today to discuss how our solutions can enhance your workflow and data integrity.
Get in touch with our experts now!
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Gold Disc Electrode
People Also Ask
- What are the key performance characteristics of a metal disk electrode? Ensuring Accurate Electrochemical Measurements
- How should a metal disk electrode be maintained? A Guide to Consistent, Reliable Electrochemical Data
- What is the typical shape and size of a metal disk electrode? A Guide to Standard and Custom Dimensions
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the purpose of selecting polycrystalline disc electrodes? Achieve Precision in Noble Metal Corrosion Research














