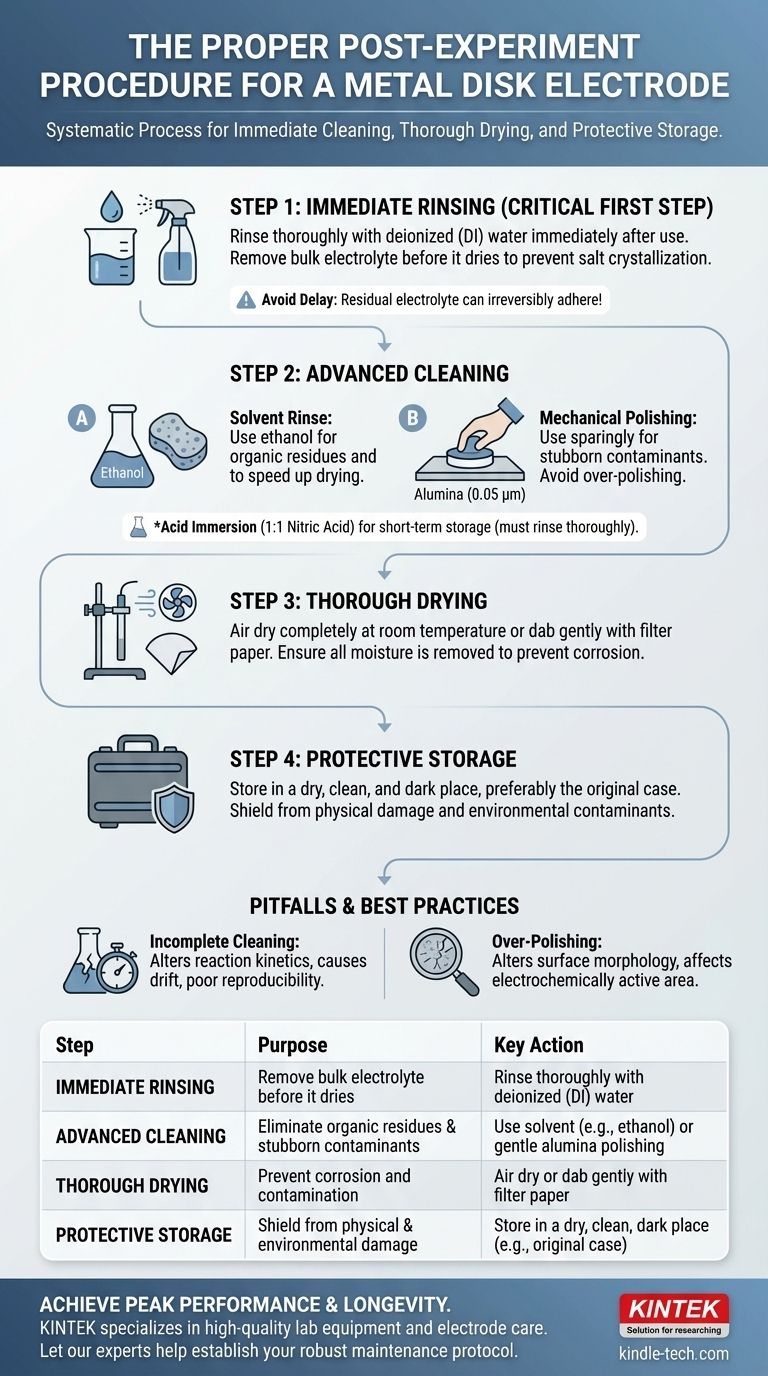
The proper post-experiment procedure for a metal disk electrode is a systematic process of immediate cleaning, thorough drying, and protective storage. This discipline is essential for preserving the electrode's sensitive surface, ensuring the accuracy and reproducibility of future measurements. The core steps involve rinsing with deionized water to remove bulk electrolyte, often followed by a solvent rinse with a substance like ethanol, and then careful drying before placing the electrode in a secure, dry environment.
The goal of post-experiment care is not just to put the electrode away, but to reset its surface to a known, clean, and reproducible state. Failing to remove all residues immediately after use can lead to irreversible contamination or surface degradation, compromising the integrity of all future experiments.

The Critical First Step: Immediate Rinsing
The moments immediately following an experiment are the most crucial for electrode maintenance. Any delay allows residual electrolyte and reaction byproducts to dry and adhere to the surface, making them significantly harder to remove.
Why Immediacy Matters
Leaving the electrode exposed to air while coated in electrolyte can cause salts to crystallize or byproducts to irreversibly adsorb onto the active surface. This creates a contaminated layer that will interfere with subsequent measurements.
The Standard Rinse: Deionized Water
The first and most important step is to thoroughly rinse the electrode with deionized (DI) water. This action removes the bulk of the electrolyte and any water-soluble species before they have a chance to dry.
Advanced Cleaning for a Pristine Surface
For many experiments, a simple water rinse is not enough. Depending on the chemical system, more advanced cleaning steps are required to fully remove all contaminants.
Using Solvents like Ethanol
After rinsing with DI water, a subsequent rinse with a solvent like ethanol is highly effective. This step helps remove organic residues or other non-polar contaminants that water alone cannot dissolve. It also helps to displace water, speeding up the drying process.
Mechanical Polishing for Stubborn Contaminants
If the electrode's performance degrades or if it was exposed to materials known to passivate the surface (like certain polymers or proteins), mechanical polishing may be necessary. This is a more intensive step and should not be part of a daily routine.
Gently polishing the electrode surface with a slurry of 0.05 µm alumina powder on a polishing pad can physically remove stubborn adsorbed layers and restore the active metal surface.
Acid Immersion for Short-Term Storage
For some applications, particularly when an electrode will be used again shortly, a brief immersion can maintain a clean surface. Immersing the electrode in a 1:1 nitric acid solution is one such method. However, the acid must be completely rinsed away with DI water before the next use.
Understanding the Pitfalls and Best Practices
An improper cleaning or storage protocol can be as damaging as the experiment itself. Understanding the risks is key to maintaining a reliable instrument.
The Risk of Incomplete Cleaning
A partially cleaned electrode is a source of experimental error. Residual contaminants can alter electrochemical reaction kinetics, cause signal drift, and lead to poor reproducibility between experiments.
The Danger of Over-Polishing
While polishing is a powerful restoration technique, overuse can be detrimental. Aggressive or frequent polishing can alter the electrode's surface morphology, change its microscopic roughness, and affect its electrochemically active surface area.
The Importance of Thorough Drying
After all rinsing and cleaning steps are complete, the electrode must be dried completely. Any remaining moisture can lead to slow corrosion or attract atmospheric contaminants. Gently dabbing with filter paper or allowing it to air dry at room temperature are common methods.
Principles of Protective Storage
Proper storage protects the electrode from both physical damage and environmental contamination. The ideal storage environment is dry, clean, and dark, away from high temperatures, humidity, and chemical fumes.
Storing the electrode in its original protective case is the simplest and most effective way to prevent the delicate surface and connection points from being damaged.
A Protocol for Your Specific Needs
Your post-experiment protocol should be tailored to the specific demands of your work.
- For routine aqueous electrochemistry: A thorough rinse with deionized water, followed by an ethanol rinse and air drying, is typically sufficient.
- If you worked with organic compounds or polymers: An ethanol or other appropriate solvent rinse is critical to remove non-polar residues that water will miss.
- If you observe passivation or poor performance: Gentle polishing with 0.05 µm alumina powder can restore the active surface, but this should be done sparingly.
- For short-term storage between frequent experiments: Immersion in a suitable cleaning solution (like 1:1 nitric acid) can be effective, but you must ensure it is completely rinsed off before the next use.
A disciplined and consistent post-experiment protocol is the foundation of reliable and reproducible electrochemical data.
Summary Table:
| Step | Purpose | Key Action |
|---|---|---|
| Immediate Rinsing | Remove bulk electrolyte before it dries | Rinse thoroughly with deionized (DI) water |
| Advanced Cleaning | Eliminate organic residues & stubborn contaminants | Use solvent (e.g., ethanol) or gentle alumina polishing |
| Thorough Drying | Prevent corrosion and contamination | Air dry or dab gently with filter paper |
| Protective Storage | Shield from physical and environmental damage | Store in a dry, clean, dark place (e.g., original case) |
Achieve peak performance and longevity for your lab's electrodes. Proper maintenance is critical for reliable data. KINTEK specializes in high-quality lab equipment and consumables, including materials for electrode care. Let our experts help you establish a robust maintenance protocol. Contact our team today to discuss your laboratory needs and ensure the integrity of your electrochemical measurements.
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Gold Disc Electrode
People Also Ask
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- How should a metal disk electrode be maintained? A Guide to Consistent, Reliable Electrochemical Data
- What is the expected lifespan of a metal disk electrode? Extend Its Life with Proper Care
- What are the key performance characteristics of a metal disk electrode? Ensuring Accurate Electrochemical Measurements
- How should a metal disk electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements



















