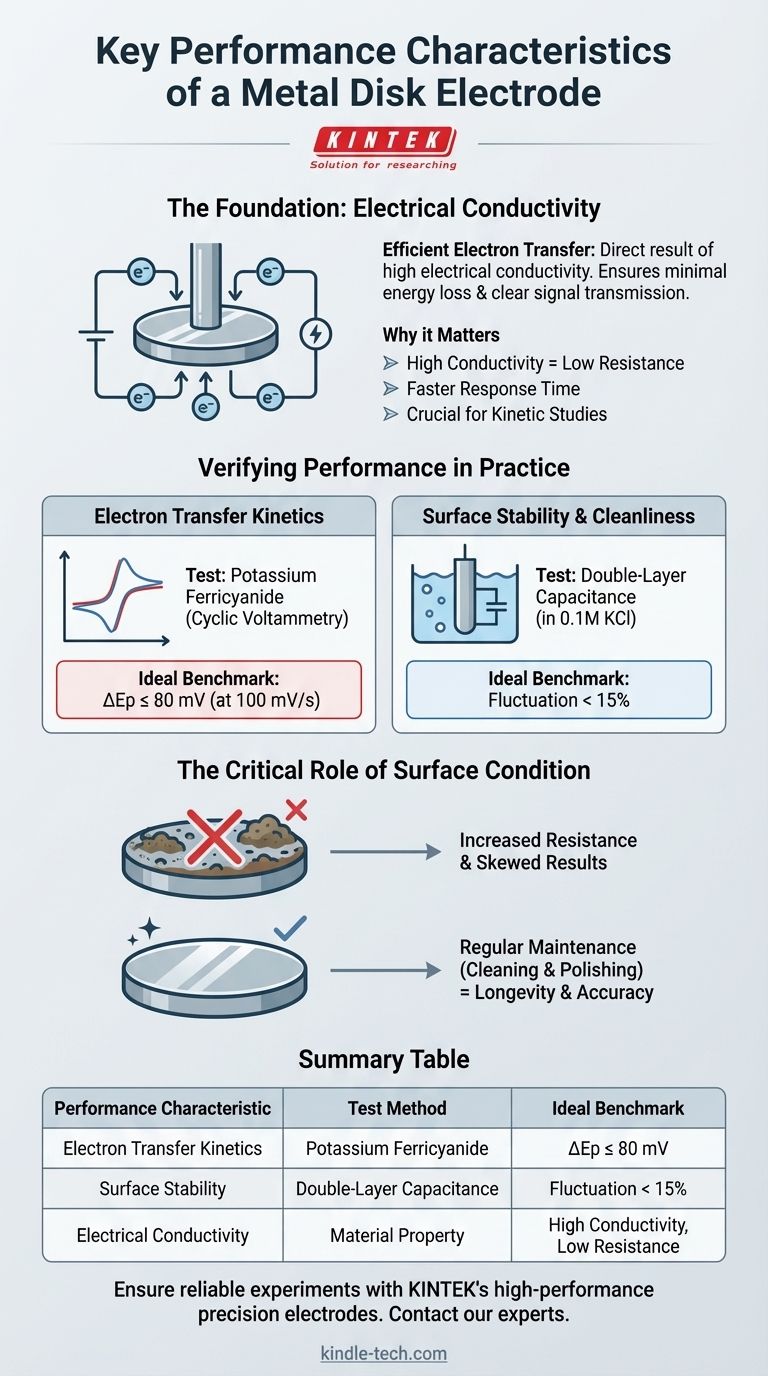
The defining performance characteristic of a metal disk electrode is its ability to facilitate efficient electron transfer, which is a direct result of its high electrical conductivity. This core property ensures minimal energy loss and clear signal transmission during an electrochemical experiment. The quality of this performance, however, is not static and depends heavily on the material's inherent properties and the cleanliness of its surface.
The true measure of a metal disk electrode's performance lies not just in its material composition but in its verifiable electrochemical behavior. Its effectiveness is confirmed through specific tests that measure electron transfer kinetics and surface stability, which are easily compromised by surface contamination.

The Foundation of Performance: Electrical Conductivity
The primary function of a working electrode is to serve as a clean interface for electron exchange between your circuit and your chemical system. High conductivity is the physical property that makes this possible.
Why High Conductivity is Essential
An electrode's conductivity dictates how easily current can flow. Materials with high conductivity, like silver or copper, allow for efficient current transfer to and from the electrolyte.
This efficiency minimizes signal interference and prevents unwanted energy loss, ensuring the data you collect accurately reflects the electrochemical reaction you are studying.
The Impact of Low Resistance
High conductivity directly translates to low electrical resistance. A low-resistance electrode enhances the speed and efficiency of signal transmission.
This results in a faster response time, which is critical for capturing the kinetics of rapid electrochemical processes.
Verifying Performance in Practice
While high conductivity is the theoretical foundation, its practical application must be verified. Two standard electrochemical tests are used to quantify the performance of a metal disk electrode.
The Potassium Ferricyanide Test
This test directly measures the electrode's ability to handle electron transfer. It is a standard cyclic voltammetry experiment using a potassium ferricyanide solution.
A key performance indicator is the peak potential separation (ΔEp). For a well-performing electrode at a scan rate of 100 mV/s, this value should be less than or equal to 80 mV. A smaller separation indicates faster, more efficient electron transfer kinetics.
The Double-Layer Capacitance Test
This measurement assesses the stability and cleanliness of the electrode's surface. It is typically conducted in a simple electrolyte solution, such as 0.1M KCl.
The performance benchmark is a fluctuation of less than 15% in the measured capacitance. A low fluctuation indicates a clean, stable surface, free from contaminants that could interfere with your primary experiment.
Common Pitfalls to Avoid
An electrode's material properties are only part of the equation. Its real-world performance is dictated by its condition at the moment of use, which can degrade over time if not properly maintained.
The Critical Role of Surface Condition
The most common point of failure is surface contamination. The presence of dirt, grease, or even a thin, invisible oxide layer can dramatically increase the electrode's resistance.
This increased resistance impedes electron transfer, which will skew your results. It often manifests as a large peak potential separation (>80 mV) in the ferricyanide test.
Longevity Depends on Maintenance
A high-quality metal disk electrode can have a long service life, but only with correct use and maintenance.
Regular cleaning and polishing are not optional; they are essential procedures required to maintain the low-resistance, high-conductivity surface needed for accurate and repeatable measurements.
Making the Right Choice for Your Goal
Ensuring your electrode is performing correctly is about validating its condition for your specific application.
- If your primary focus is kinetic studies: Verify performance with the potassium ferricyanide test to ensure your ΔEp is below 80 mV, confirming rapid electron transfer.
- If your primary focus is stable, long-term measurements: Use the double-layer capacitance test to confirm your surface is clean and stable, with fluctuations below 15%.
Ultimately, understanding these performance characteristics allows you to control the quality of your most critical experimental tool.
Summary Table:
| Performance Characteristic | Test Method | Ideal Benchmark |
|---|---|---|
| Electron Transfer Kinetics | Potassium Ferricyanide (Cyclic Voltammetry) | ΔEp ≤ 80 mV at 100 mV/s |
| Surface Stability & Cleanliness | Double-Layer Capacitance (in 0.1M KCl) | Fluctuation < 15% |
| Electrical Conductivity | Material Property (e.g., Silver, Copper) | High Conductivity, Low Resistance |
Ensure your electrochemical experiments are built on a reliable foundation. KINTEK specializes in high-performance lab equipment and consumables, including precision electrodes designed for superior conductivity and durability. Whether you're conducting kinetic studies or long-term measurements, our products help you achieve accurate, repeatable results. Contact our experts today to find the perfect electrode solution for your laboratory needs.
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Gold Disc Electrode
People Also Ask
- How should a metal disk electrode be maintained? A Guide to Consistent, Reliable Electrochemical Data
- What is the expected lifespan of a metal disk electrode? Extend Its Life with Proper Care
- How should a metal disk electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What is the typical shape and size of a metal disk electrode? A Guide to Standard and Custom Dimensions
- What methods can be used to verify the performance of a metal disk electrode? Ensure Accurate Electrochemical Results



















